
Research Branch (ÖSTAT Classification)
302054, 301904, 301206
Keywords
hormone refractory prostate cancer, neuroendocrine tumours, peptide ligand radionuclide therapy, Peptide receptor radionuclide therapy, radioiodine refractory thyroid cancer, and thera(g)nostics
Research Focus
The Department of Nuclear Medicine is best known for its work with radiolabelled peptides, both for diagnostic and for therapeutic purposes, a theme that we have systematically explored over the last three decades. We are developing a variety of radiopharmaceuticals for different targets for clinical use. Our goal is to engineer more effective ligands/peptides/antibodies- “thera(g)nostics”- for individualised treatment, mainly in the area of oncology.
General Facts
The Department of Nuclear Medicine accelerates the translation of preclinical radiopharmaceutical research development (focus on radiolabelled peptides) into clinical applications, towards imaging of biomarkers used for cancer treatment (70% of clinical routine), treatment of neurological impairment (20% of clinical routine) and cardiac disease (10% of clinical routine). The structure of the Department of Nuclear Medicine is based on a very creative, productive, well-funded and internationally respected, high-quality, preclinical research and development unit. This group consists of several radiochemists/pharmacists, medical physicists and PhD-students. Their work results in the construction of radiotracers, which use different modal systems including a variety of radiolabelled peptide analogues such as for somatostatin, vasoactive intestinal peptide (VIP), cholecystokinin (CCK-2/gastrin) and prostate-specific membrane antigen (PSMA) ligand for specific tumour targeting. Other important developments are based on Arg-Gly-Asp (RGD) for imaging of angiogenesis in tumour lesions and on hepatic binding protein imaging with galactosylated albumin (NGA) for functional liver reserve estimation. Radiopharmaceuticals are produced at clinical grade in our dedicated laboratories, for use in SPECT/CT or PET/CT studies.
About 30 whole-body PET/CT studies are performed daily in our PET centre. Patients assessed by SPECT/CT dosimetry studies are treated in our Nuclear Medicine Therapy ward with high-dose thera(g)nostics. Radioiodine ablation therapy of thyroid cancer remnants, peptide receptor radionuclide therapy (PRRT) of neuroendocrine tumour patients and peptide ligand radionuclide therapy (PLRT) of prostate cancer patients are currently our most important therapy tools. The Department of Nuclear Medicine is involved not only in many clinical studies with radiolabelled thera(g)nostics, particularly industry-sponsored Phase I/IIa trials, but also in academic Phase I trials with radiopharmaceuticals developed in-house.
Research
The research activities are focused on preclinical research dedicated to the optimisation and improvement of radiolabelling procedures for established radiopharmaceuticals, the in-house preparation of new radiopharmaceuticals for clinical studies and the preclinical development of new radio ligands for molecular imaging and therapeutic purposes. Different research projects illustrate the activities in this field. Some of the thera(g)nostics developed in the Department of Nuclear Medicine have already entered larger clinical Phase I/II trials.
Siderophores for Molecular Imaging and Thera(g)nostics
Leads: Clemens Decristoforo and Bernhard Nilica
Nuclear Medicine can play a vital role in the field of infection, through molecular imaging and thera(g)nostics. Siderophores are low-molecular mass, Fe3+-specific chelators secreted by bacteria and fungi. The use of siderophores labelled with gallium-68 for PET has been a focus of preclinical research for many years. In a current, preclinical project, Triacetylfusarinine C (TAFC) – the main extracellular siderophore of Aspergillus fumigatus (AFU), responsible for severe invasive fungal infections – is modified for novel applications. The main objective is to develop and characterise novel analogues of TAFC targeting AFU in vivo and to establish a novel concept for “thera(g)nostics” (combining diagnosis and therapy) of invasive aspergillosis. One example is the chemical modification of TAFC with fluorescent dyes (PhD thesis of Joachim Pfister). This allows so-called “hybrid imaging” by combining optical imaging (650 – 800 nm excitation wavelength) with PET. An example of microscopy of such a compound and in vivo imaging in a rat model infected with AFU in the lung by PET/CT and optical imaging is shown in Fig. 1 [1].
![Fig.1: Coronal PET/CT slices of immunocompromised Lewis-rats infected with A. fumigatus in the lung using 68Ga-labelled antifungal “theranostic agents” based on siderophores. Images are showing the lung section of infected (top row) and non-infected (bottom row, control) rats of each compound, respectively. CT images were added to show the severity of the infected lung tissue. Images reproduced from Pfister et al. [1]. Further developments are currently pursued including the use of modified Ferioxamines or the development of artificial siderophores for molecular imaging in close cooperation with H. Haas (Biocenter) and the University of Wrozlaw, inorganic chemistry (E. Gumienna-Kontecka) [2].](https://researchreport.i-med.ac.at/wp-content/uploads/2023/09/Fig.1--1024x680.jpg)
Based on the successful preclinical development of 68Ga-labelled siderophores, translation into clinical practice has been initiated for PET infection imaging. A Phase I/IIa clinical trial using the clinically approved drug deferoxamin (Desferal ®) labelled with Gallium-68 for imaging bacterial infections was submitted and approved by the ethics committee and regulatory authority (EudraCT no.: 2020-002868-31). This clinical trial, led by Bernhard Nilica and starting in 2021, is supported by FWF within the KLIF-track (Proj. no. KLI 909-B to C. Decristoforo).
Development of Multimodality Imaging probes
Lead: Clemens Decristoforo
Based on the success using radio-labelled Siderophores for imaging of Infection, novel targeted agents for molecular imaging were developed based on cyclic sideophore based scaffolds by coupling a variety of targeting sequences in a recent FWF project. In particular this approach allows the combination of different imaging modalities, in particular PET with optical imaging approaches using one “hybrid” imaging probe. This would potentially allow the combination of whole body PET imaging with optical guided surgery. Within the FWF funded PhD programme IGDT- ART (https://phd-igdt-art.i-med.ac.at/) the PhD student Giacomo Gariglio is working on the development of novel probes combining a cyclic scaffold (being able to form stable bonds with radio metals such as Gallium-68 for PET imaging) with a tumour targeting sequence and an optical dye. First promising results have already been achieved in the initial phase of this project.
Cholecystokinin Receptor Thera(g)nostics
Lead: Elisabeth von Guggenberg, Christian Uprimny, Gianpaolo Di Santo & Irene Virgolini
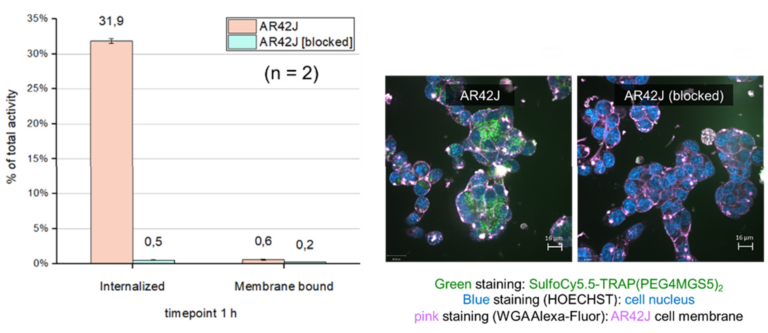
Radiolabelled minigastrin (MG) analogues specifically binding to the cholecystokinin-2 receptor (CCK2R) provide a very sensitive molecular imaging technique for the detection of medullary thyroid carcinoma (MTC) and other CCK2R-expressing malignancies, such as gastroenteropancreatic and bronchopulmonary NET [Fig 2].
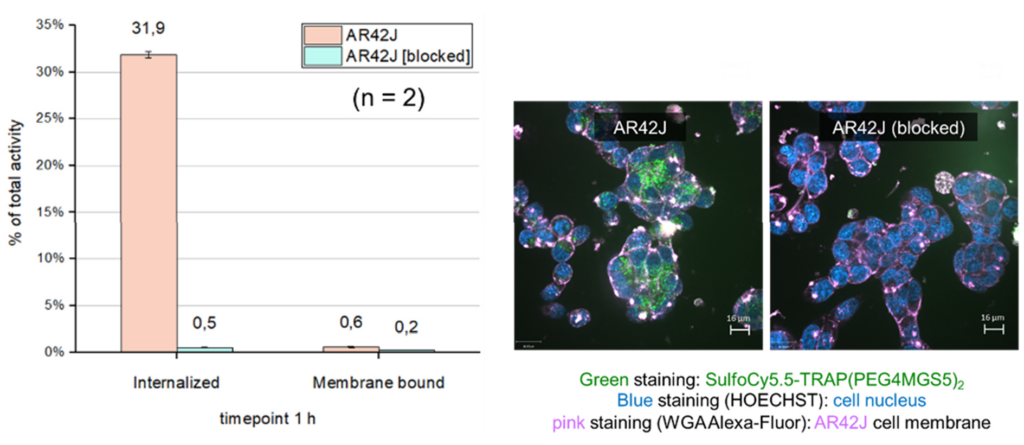
When radiolabelled with ß- emitting radionuclides, these analogues should become useful for targeted radiotherapy [3]. New radiolabelled peptide analogues have been developed to overcome high kidney uptake and low metabolic stability, which especially limit the therapeutic application of the currently available radiolabelled minigastrin analogues. By exploring novel stabilisation strategies within the receptor-binding motif of the linear amino acid sequence, it has been possible to develop novel minigastrin analogues with improved stability and retained receptor binding. From a pool of twelve different new peptide analogues developed within the FWF project P27844, DOTA- MGS5 was selected as new lead compound for further development. Conjugation to the macrocyclic chelator DOTA enables stable radiolabelling with different radioisotopes, such as indium-111 for SPECT, gallium-68 for PET and lutetium-177 for therapeutic use. For DOTA- MGS5 labelled with the different radio metals, a high and comparable tumour uptake was observed in the mouse tumour xenograft model [4]. A first proof of principle of high-sensitivity PET/CT imaging was achieved for 68Ga-DOTA-MGS5 in a 75-year-old female patient with recurrent MTC
![Fig 3: PET CT imaging in a patient with recurrent MTC using [18F]FDOPA (a, d, f, h) and [68Ga]Ga-DOTA-MGS5 (b/c, e, g, i – l), showing several pathologic lesions suggestive of metastases (red arrows: lymph nodes; blue arrows: bone lesions; green arrows: liver lesions); three liver lesions were undetectable by standard imaging with [18F]F-DOPA. Images reproduced from [5].](https://researchreport.i-med.ac.at/wp-content/uploads/2023/09/Fig-3-2.jpg)
In the new FWF project P34732, the postdoc Anton Hörmann evaluates new innovative radiolabelling strategies for high sensitivity PET imaging of cholecystokinin-2-receptor expressing tumours. Within the project, alternative peptide conjugates with elongated or truncated sequence conjugated to different bifunctional chelators will be investigated. Furthermore, two attractive radiofluorination strategies will be explored. Within the FWF funded DOC 110 doc.funds programme IGDT-ART the PhD student Taraneh Zavvar is proceeding with the clinical translation of peptide receptor radionuclide therapy targeting CCK2R. A clinically suitable radiopharmaceutical formulation will be developed and all the documents for the clinical trial application will prepared for a first clinical trial evaluating the safety of administration as well as dosimetry aspects of a 177Lu-labelled minigastrin analogue.
Angiogenesis Imaging – Non-invasive Determination of the Integrin αvβ3 Expression
Leads: Roland Haubner & Gianpaolo di Santo
Tumour-induced angiogenesis is a key process in the development of cancer. One receptor involved in this complex process is integrin αvβ3. We developed a 68Ga-labelled small cyclic peptide ([68Ga]NODAGA-RGD), which binds with high affinity to this receptor [7]. Based on the very promising preclinical results and on the initial data (Fig.4)
![Fig 4: Maximum-intensity projections from static [68Ga] NODAGA-RGD PET scans of a male patient with hepatocellular carcinoma at 13 min (A), 40 min (B), and 76 min (C) after tracer injection. The tracer shows rapid predominant renal elimination with the highest activity in the bladder, kidneys, liver, spleen and intestine. Low background activity is found in the brain, thorax and extremities, resulting in a low radiation dose for the patient. For all three images, the grey-scale is set to the same value. Image from [8]](https://researchreport.i-med.ac.at/wp-content/uploads/2023/09/Fig-4-1024x347.jpg)
Non-Invasive Determination of the Functional Liver Reserve
Leads: Roland Haubner &Irene Virgolini
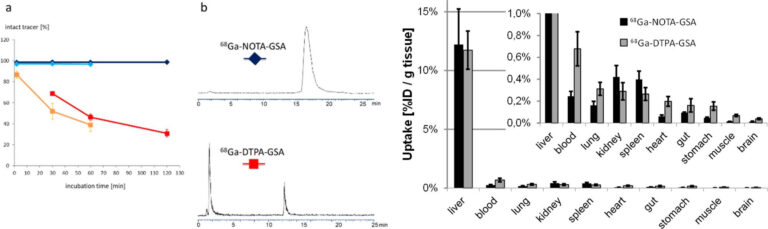
The asialoglycoprotein receptor (ASGR) is exclusively expressed on the basolateral side of hepatocytes, which makes it a promising target for drug delivery into hepatocytes. Moreover, due to its restricted expression, the ASGR is an optimal target structure for non-invasive monitoring of the functional liver reserve. Our initial developments resulted in 68Ga-NOTA- GSA, which demonstrated high metabolic stability and confirmed the superiority of PET for imaging the functional liver reserve [9] (Fig. 5).
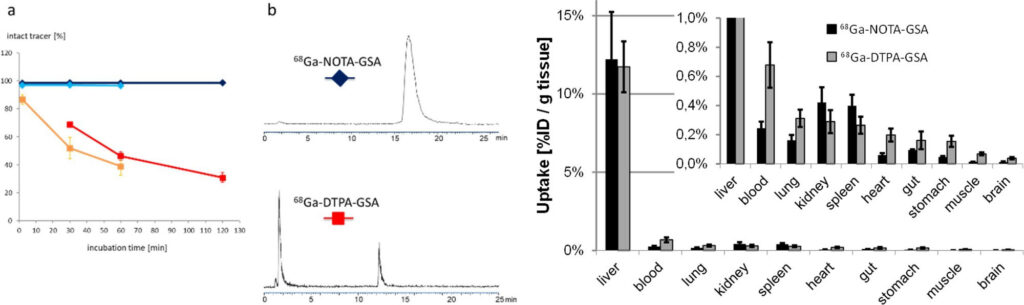
Unfortunately, the requirements for production of a GMP-compliant labelling precursor have increased in recent years. Consequently, despite the good imaging performance, translation of this human serum albumin-based labelling precursor into clinical practice has not been possible, which indicates the need for radiolabelled synthetic derivatives of low molecular weight as galactose carriers. To satisfy these requirements, the development of such derivatives has been started and a corresponding proposal for funding has been submitted to FWF (P 34802-B).
Because of the metabolic stability, activity concentration in blood and most other organs is even lower than for the lead structure. Images reproduced from [9].
Novel Radionuclides for Thera(g)nostic Applications (PRISMAP Project)
Lead: Clemens Decristoforo
The goal of PRISMAP (www.prismap.eu, funded by the Horizon 2020 research and innovation programme, grant agreement No 101008571) is to provide a sustainable source of high purity grade new radionuclides for medicine, involving from the onset upcoming major European infrastructures, to provide a single-entry point for all researchers active in this field including SMEs, global pharma, nuclear centres, hospitals and universities, using standardised access procedures. The new isotope enrichment and standardisation techniques triggered in PRISMAP will expand services and provide them to yet unreachable remote European laboratories. PRISMAP thus strives to create a paradigm shift in the early phase research on radiopharmaceuticals, targeted drugs for cancer – one of the major diseases in Europe – theranostics and personalised medicine, shaping the European isotope landmark as a gold standard to accelerate the development of the pharma industry and ultimately of a better healthcare for the improvement of our citizens’ life. The department of Nuclear Medicine leads Workpackage 4 of the project with the aim to harmonise the pharmaceutical standards required for clinical translation of innovative radionuclides. A first guidance document has recently been published [10].
Novel Technetium-99m labelled Somatostatin Antagonists – TECANT Project
Leads: Irene Virgolini, Gianpaolo Di Santo & Clemens Decristoforo
The overexpression of somatostatin receptors (SSTR) predominantly of subtype – 2 (SSTR2) in neuroendocrine neoplasms is an established target for radiopharmaceuticals, which allows prediction and evaluation of response to various available therapies. The development of a receptor antagonist labelled with technetium-99m for SPECT with superior imaging properties is the aim of the ERA-PerMED project “TECANT” [https://www.era-learn.eu/network- information/networks/era-permed/1st-joint-transnational-call-for-proposals-2018/] supported by FWF (proj. no. I 4220-B). SSTR antagonists were prepared and compared pre-clinically in respect of their pharmacological properties in vitro and in vivo to provide a basis for clinical translation. [99mTc]-TECANT 1, was selected as the best candidate. Fig. 6
![Fig. 6: The Era-PERMED TECANT project scheme, with a focus on WP1, probe selection as with substantial contribution from the MUI Department of Nuclear Medicine, reproduced from [11]](https://researchreport.i-med.ac.at/wp-content/uploads/2023/09/Fig.-6-1-1024x452.jpg)
NeoFind, Neother and NeoRay Clinical Studies
Leads: Clemens Decristoforo, Bernhard Nilica, Gianpaolo Di Santo, Christine Rangger &Irene Virgolini
Based on an EU FP7 project Mitigate (FP7 No 602306), the Department of Nuclear Medicine performed the first diagnostic in vivo studies with a novel 68Ga-labelled Bombesin antagonist for PET in patients with GIST tumours (“A Phase I/IIa study to evaluate safety, biodistribution, dosimetry and preliminary diagnostic performance of 68Ga-NeoBOMB1 in patients with advanced TKI-treated GIST using positron-emission tomography/computer tomography (PET/CT).” EudraCT: 2016-002053-38, [13,14]. Consequently, the industry-sponsored (AAA/Novartis) NeoFIND study (“Phase II study of preliminary diagnostic performance of [68Ga]-NeoBOMB1 in adult patients with malignancies known to overexpress gastrin releasing peptide receptor” (EudraCT: 2017-003432-37) was implemented in Innsbruck and resulted in the NeoTher study “Phase Ia-Ib, safety, tolerability, whole-body distribution, radiation dosimetry of [177Lu]-NeoBOMB1 administered in subjects with gastrin-releasing peptide receptor (GRPR) positive relapsed or refractory metastatic breast and prostate cancer” and in the NeoRay study (“A Phase I/IIa open-label, multi-centre study to evaluate the safety, tolerability, whole-body distribution, radiation dosimetry and anti-tumour activity of [177Lu]-NeoB administered in patients with advanced solid tumours known to overexpress gastrin-releasing peptide receptor (GRPR).” (EudraCT: 2018-004727-37), which is currently recruiting tumour patients.
Prostate Cancer Thera(g)nostics
Lead: Christian Uprimny, Gianpaolo Di Santo & Irene Virgolini
New receptor radiotracers binding to the prostate-specific membrane antigen (PSMA) – which is significantly increased in prostate cancer (PC) cells – have been proposed for PET imaging. The 68Ga-PSMA ligand HBED-CC has proven its feasibility for the detection of PC relapses and metastases with high sensitivity. In recent years, many clinical studies have demonstrated the great potential of 68Ga-PSMA PET/CT in patients with biochemical relapse [15-18]. Our recent multicentre analysis for patients with PSA relapse has shown significant differences in overall survival for patient groups with PSA < 0.2, 0.2-0.5, 0.51-1.0 and > 1.0 ng/ml before restaging with 68Ga-PSMA-PET/CT (Fig. 7).
![Fig. 7: Image is reproduced from [18]](https://researchreport.i-med.ac.at/wp-content/uploads/2023/09/Fig.-7.jpg)
Based on the high-level expression of PSMA in PC cells we have started to use high-dose 177Lu-PSMA ligand to treat patients who have metastasised disease [Fig 8].
![Fig. 8: On the PET-scan prior to therapy local tumour in the prostate bed (red arrow), multiple abdominopelvic and one cervical LN metastases (green arrows) were clearly visible on maximum intensity projection (MIP) (1a) and on fused axial PET/CT-images (1b, 1c). Restaging PET/CT performed eight weeks after administration of 4 cycles of 177Lu-PSMA- 617 with a total accumulated activity of 24.93 GBq showed a markedly reduction of the primary tumour (red arrow) and an impressive partial response of LN-metastases with only small metastases left in the right iliac region (green arrows), as displayed on MIP (2a) and fused axial PET/CT-images (2b, 2c). Image reproduces from [19].](https://researchreport.i-med.ac.at/wp-content/uploads/2023/09/Fig.-8-1024x415.jpg)
Somatostatin Receptor Thera(g)nostics
Leads: Margarida Rodrigues, Anna Sviridenko & Irene Virgolini
Peptide receptor RT (PRRT) has been recognised as a promising treatment for neuroendocrine (NET) patients. The use of 18F-FDG PET in NET is still controversial, whereas 68Ga-labelled somatostatin analogues are recognised radiopharmaceuticals. The long-term survival and efficacy of a second PRRT course with somatostatin analogues 177Lu-DOTATE in patients with advanced gastroenteropancreatic NET was evaluated (thesis of Kevin Winkler). Furthermore, the value of 18F-FDG PET/CT in these patients was assessed. Forty patients with GEP NET, who underwent two PRRT courses with 177Lu-DOTATATE and combined examinations with 68Ga-DOTA-TOC and 18F-FDG PET/CT, were evaluated (Fig. 9).
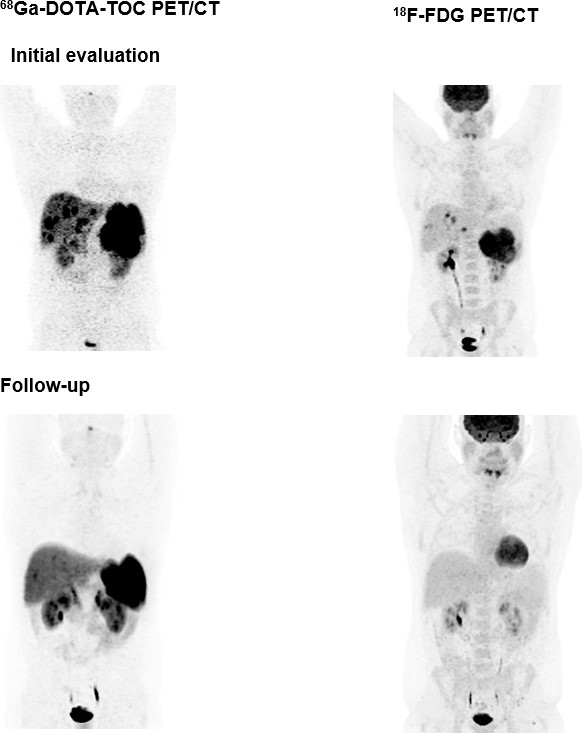
After the second PRRT course, 2 patients (5.0%) were in partial remission, 21 patients (52.5%) experienced stable disease and 17 patients (42.5%) had progressive disease. The median overall survival was 122 months. After the second PRRT course, the median overall survival was significantly higher (P=0.033) in the 18F-FDG-negative group compared with the 18F-FDG- positive group (145.50 versus 95.06 months, respectively). The median time to progression was 19.37 months. In conclusion, a second PRRT course with 177Lu-DOTATE is an effective treatment approach for GEP NET patients with disease progression. A change in 18F-FDG status after PRRT may predict disease course and survival. Patients who are 18F-FDG negative have significantly longer overall survival than those who are 18F-FDG positive [22].
18F-FDG positivity provides the basis for a combination of PRRT with chemotherapy (in collaboration with the oncology department) and the patients are being treated with this combination scheme at the Department of Nuclear Medicine.
Over the last two decades, various somatostatin analogues have been implemented for thera(g)nostic use. The IPSEN-sponsored clinical studies using a somatostatin antagonist were:
“A multicentre, randomized, dose-confirmation, factorial Phase 2 study to evaluate the optimal dose of 68Ga-OPS202 as a PET imaging agent in subjects with gastroenteropancreatic neuroendocrine tumour (GEPNET)” EudraCT: 2016-004928-39.
68Ga-OPS202 in Breast Cancer Patients: “A non-randomized Phase ll study to evaluate the optimal uptake time of 68Ga-OPS202 as a sstr2 positive PET imaging agent in subjects with newly diagnosed breasts cancer.” EudraCT: 2018-000028-33/NCT.
“A multicentre, open-label Phase l/ll study to evaluate the safety, tolerability, biodistribution and anti-tumour activity of 177Lu-OPS201 with companion imaging 68Ga-OPS202 PET/CT in previously treated subjects with locally advanced or metastatic cancers expressing somatostatin receptor 2 (SSTR2)” EudraCT: 2017-005173-39/NCT.
Neurotensin Receptor Imaging
Leads: Irene Virgolini & Feng Wand, Nanjing, China
In collaboration with Professor Feng Wang from Nanjing in China (where Irene Virgolini is an appointed Visiting Professor), initial 68Ga-labelled-Neurotensin analogue- PET/CT scans were performed on patients with pancreatic cancer [23]. The positive scans point to a future role for such analogues and possibly to identification of a stable 177Lu-labelled neurotensin analogue for therapy.
Fibroblast Activation Protein (FAP) inhibitor imaging
Leads: Anna Sviridenko & Irene Virgolini
In recent years the imaging of FAP-expression on fibroblasts has become a tool in oncologic patients using 68Ga-labelled inhibitors. We used 68Ga-FAPI-46 in the follow-up of SARS-COV2- infected patients [24]. First studies indicated a potential role for FAP-Imaging in impaired pulmonary convalescence.
![Fig. 10: Comparison of 18F-FDG PET/CT (A–C) and 68Ga-FAPI-46 PET/CT (D–F): the corresponding transaxial low-dose CT scans (A and D) and PET emission scans (B and E), together with the coronal MIPs (C and F). Showing no relevant accumulation of FDG but an accumulation of FAPI-46, 19 weeks after discharge from hospital, in residual peripheral ground-glass opacities and subtle reticular changes shown in the corresponding low dose CT scans. In addition, a serial rib fractures right and inflammatory changes in left hip were detected on both scans. Reproduced from [24].](https://researchreport.i-med.ac.at/wp-content/uploads/2023/09/Fig.-10.jpg)
Pictures
Selected Publications
- Pfister J, Petrik M, Bendova K, Matuszczak B, Binder U, Misslinger M, a. Antifungal Siderophore Conjugates for Theranostic Applications in Invasive Pulmonary Aspergillosis Using Low-Molecular TAFC Scaffolds. JoF. 2021;7(7):558.
- Mular A, Shanzer A, Kozłowski H, Hubmann I, Misslinger M, Krzywik J, a. Cyclic Analogs of Desferrioxamine E Siderophore for 68Ga Nuclear Imaging: Coordination Chemistry and Biological Activity in Staphylococcus aureus. Inorg Chem. 2021;60(23):17846–57.
- Klingler M, Hörmann AA, Guggenberg Cholecystokinin-2 Receptor Targeting with Radiolabeled Peptides: Current Status and Future Directions. Curr Med Chem 2020, 27, 7112-7132
- Klingler M, Summer D, Rangger C, Haubner R, Foster J, Sosabowski J, Virgolini I, Decristoforo C, von Guggenberg DOTA-MGS5, a new cholecystokinin-2 receptor targeting peptide analog with optimised targeting profile for theranostic use. J Nucl Med. 2019 Jul;60(7):1010-1016.
- Uprimny C, von Guggenberg E, Svirydenka A, Mikołajczak R, Hubalewska-Dydejczyk A, Virgolini Comparison of PET/CT imaging with [18F]FDOPA and cholecystokinin- 2 receptor targeting [68Ga]Ga-DOTA-MGS5 in a patient with advanced medullary thyroid carcinoma. Eur J Nucl Med Mol Imaging. 2021 Mar;48(3):935-936. doi: 10.1007/s00259-020-04963-z. Epub 2020 Aug 3. PMID: 32748048.
- von Guggenberg E, Uprimny C, Klingler M, Warwitz B, Sviridenko A, Bayerschmidt S, Di Santo G, Virgolini Preliminary clinical experience of cholecystokinin-2 receptor PET/CT imaging using the 68Ga-labeled minigastrin analog DOTA-MGS5 in patients with medullary thyroid cancer. J Nucl Med. 2023 Jan 19:jnumed.122.264977. doi: 10.2967/jnumed.122.264977. Epub ahead of print. PMID: 36657979.
- Knetsch PA, Petrik M, Griessinger CM, Rangger C, Fani M, Kesenheimer C, von Guggenberg E, Pichler BJ, Virgolini I, Decristoforo C, Haubner [68Ga]NODAGA-RGD for imaging αvβ3 integrin expression. Eur J Nucl Med Mol Imaging 2011, 38:1303-1312
- Haubner R, Finkenstedt A, Stegmayr A, Rangger C, Decristoforo C, Zoller H, Virgolini [68Ga]NODAGA-RGD – Metabolic stability, biodistribution, and dosimetry data from patients with hepatocellular carcinoma and liver cirrhosis. Eur J Nucl Med Mol Imaging. 2016; 43:2005-2013.
- Haubner R, Schmid AM, Maurer A, Rangger C, Roig LG, Pichler BJ, Virgolini [68Ga]NOTA-Galactosyl Human Serum Albumin: a Tracer for Liver Function Imaging with Improved Stability. Mol Imaging Biol. 2017; Feb 13. doi: 10.1007/s11307-017- 1046-1.
- PRISMAP- Standards for clinical translation, DOI 5281/zenodo.6599180.
- Fani M, Weingaertner V, Kolenc Peitl P, Mansi R, Gaonkar RH, Garnuszek P, Mikolajczak R, Novak D, Simoncic U, Hubalewska-Dydejczyk A, Rangger C, Kaeopookum P, Decristoforo Selection of the First 99mTc-Labelled Somatostatin Receptor Subtype 2 Antagonist for Clinical Translation-Preclinical Assessment of Two Optimised Candidates. Pharmaceuticals (Basel). 2020 Dec 28;14(1):19. doi: 10.3390/ph14010019.
- Novak D, Janota B, Hörmann AA, Sawicka A, Kroselj M, Hubalewska-Dydejczyk A, Fani M, Mikolajczak R, Kolenc P, Decristoforo C, Garnuszek Development of the 99mTc-Labelled SST2 Antagonist TECANT-1 for a First-in-Man Multicentre Clinical Study. Pharmaceutics. 2023 Mar 9;15(3):885. doi: 10.3390/pharmaceutics15030885
- Gruber L, Jiménez-Franco LD, Decristoforo C, Uprimny C, Glatting G, Hohenberger P, Schoenberg SO, Reindl W, Orlandi F, Mariani M, Jaschke W, Virgolini MITIGATE- NeoBOMB1, a Phase I/IIa Study to Evaluate Safety, Pharmacokinetics and Preliminary Imaging of 68Ga-NeoBOMB1, a Gastrin-Releasing Peptide Receptor Antagonist, in GIST Patients. J Nucl Med. 2020 Dec;61(12):1749-1755. pii: jnumed.119.238808. doi: 10.2967/jnumed.119.238808. Epub 2020 Apr 24.
- Gruber L, Decristoforo C, Uprimny C, Hohenberger P, Schoenberg S, Orlandi F, Mariani M, Manzl C, Kasseroller MT, Tilg H, Zelger B, Jaschke W, Virgolini I. Tumour Targeting and Imaging Properties of 68 Ga- NeoBOMB1, a gastrin-releasing peptide receptor antagonist, in GIST Biomedicines. 2022 Nov 11;10(11):2899. doi: 10.3390/biomedicines10112899.
- Uprimny C, Bayerschmidt S, Kroiss AS, Fritz J, Nilica B, Svirydenka A, Decristoforo C, Di Santo G, von Guggenberg E, Horninger W, Virgolini Impact of forced diuresis with furosemide and hydration on the halo artefact and intensity of tracer accumulation in the urinary bladder and kidneys on [68Ga]Ga-PSMA-11-PET/CT in the evaluation of prostate cancer patients. Eur J Nucl Med Mol Imaging. 2020 May 8. doi: 1.1007/s00259-020-04846-3
- von Eyben R, Knapp D, Hoffamann M, Soydal S, Uprimny C, Virgolini I, Tuncel M, Gauthe M, von Eyben A PSMA PET/CT risk model for overall survival for patients with prostate cancer at PSA relapse. Cancers 2022, 14(21), 5461; https://doi.org/10.3390/cancers14215461
- von Eyben FE, Bauman G, Kapp DS, Virgolini I, Paganelli On the Way for Patients with Prostate Cancer to the Best Use of PSMA. Int J Mol Sci. 2022 Feb 24;23(5):2478. doi: 10.3390/ijms23052478. PMID: 35269620; PMCID: PMC8909989.
- von Eyben R, Hoffmann MA, Kapp DS, Soydal C, Uprimny C, Virgolini I, Tuncel M, Gauthé M, von Eyben Quality Goal for Salvage Treatment for Patients with Prostate Cancer at Prostate-specific Antigen Relapse. Eur Urol Oncol. 2022 Feb 5:S2588- 9311(22)00006-2. doi: 10.1016/j.euo.2022.01.005. Epub ahead of print. PMID: 35135731.
- Maffey-Steffan J, Scarpa L, Svirydenka A, Bernhard N, Mair C, Buxbaum S, Bektic J, vonGuggenberg E, Uprimny C, Horninger W, Virgolini The 68Ga/177Lu-theragnostic concept in PSMA-targeting of metastatic castration-resistant prostate cancer: impact of post-therapeutic whole-body scintigraphy in the follow-up. Eur J Nucl Med Mol Imaging. 2020 Mar;47(3):740.
- Ahmadzadehfar H, Rahbar K, Baum R, Seifert R, Kessel K, Bögemann M, Kulkarni HR, Zhang J, Gerke C, Fimmers R, Kratochwil C, Rathke H, Ilhan H, Maffey-Steffan J, Sathekge M, Kabasakal L, Garcia-Perez FO, Kairemo K, Maharaj M, Paez D, Virgolini
- Prior therapies as prognostic factors of overall survival in metastatic castration- resistant prostate cancer patients treated with [177Lu]Lu-PSMA-617. A WARMTH multicentre study (the 617 trial). Eur J Nucl Med Mol Imaging. 2020 May 8. doi: 10.1007/s00259-020-04797-9.
- Ahmadzadehfar H, Matern R, Baum RP, Seifert R, Kessel K, Bögemann M, Kratochwil C, Rathke H, Ilhan H, Svirydenka H, Sathekge M, Kabasakal L, Yordanova A, Garcia- Perez FO, Kairemo K, Maharaj M, Paez D, Virgolini I, Rahbar The impact of the extent of the bone involvement on overall survival and toxicity in mCRPC patients receiving 177Lu-PSMA-617: a WARMTH multicentre study. Eur J Nucl Med Mol Imaging. 2021; 48: 4067-76. doi: 10.1007/s00259-021-05383-3.
- Rodrigues M, Winkler KK, Svirydenka H, Nilica B, Uprimny C, Virgolini Long-term survival and value of 18F-FDG PET/CT in patients with gastroenteropancreatic neuroendocrine tumors treated with second peptide receptor radionuclide therapy cycle with 177Lu-DOTATATE. Life. 2021; 11: 198-10; doi: 10.3390/life11030198.
- Hodolic M, Wu WY, Zhao Z, Yu F, Virgolini I, Wang Safety and tolerability of 68Ga- NT-20.3, a radiopharmaceutical for targeting neurotensin receptors, in patients with pancreatic ductal adenocarcinoma: the first in-human use. Eur J Nucl Med Mol Imaging. 2021 Apr;48(4):1229-1234. doi: 10.1007/s00259-020-05045-w. Epub 2020 Oct 2. PMID: 33006657.
- Sviridenko A, Boehm A, di Santo G, Uprimny C, Nilica B, Fritz J, Giesel FL, Haberkorn U, Sahanic S, Decristoforo C, Tancevski I, Widmann G, Loeffler-Ragg J, Virgolini Enhancing Clinical Diagnosis for Patients With Persistent Pulmonary Abnormalities After COVID-19 Infection: The Potential Benefit of 68 Ga-FAPI PET/CT. Clin Nucl Med. 2022 Dec 1;47(12):1026-1029. doi: 10.1097/RLU.0000000000004437. Epub 2022 Oct 15. PMID: 36257062; PMCID: PMC9653058.
Selection of Funding
- FWF (P30924-B26) Modified Siderophores for “Theranostics” of Aspergillosis 2018 Clemens Decristoforo/ –
- FWF (KLI 909-B) 68Ga-DFO based PET-Imaging of Infections, 2021 Dr.Clemens Decristoforo/183.965.-
- FWF (I4229-B) Novel 99mTc-labeled somatostatin receptor antagonists 2019 Dr.Irene Virgolini/230842.-
- PRISMAP The European medical isotope programme: Production of high purity isotopes by mass separation EU-INFRAIA, H2020 March 2021-February 2025 (C. Decristoforo)/261500.-
- DOC-Funds: Image-guided Diagnosis and Therapy (IGDT): Integrating multimodal strategies for clinical research FWF 2021-2025 (C. Decristoforo, E. von Guggenberg)/ 371.540.-
- Exploration of innovative radiolabelling strategies for high sensitivity PET imaging of cholecystokinin-2-receptor expressing tumours Innovative radiolabelling strategies for CCK2R targeting FWF P 34732 (E. von Guggenberg) 2021-2024/371.505.-
- New PET tracer for imaging of functional liver FWF P 34802 (R. Haubner) 2021/Euro 388336.-
Collaborations
- Prof. Stefano Fanti, Policlinico S.Orsola-Malpighi, Bologna, Italia
- Prof. Dr. Marcus Hacker, Medical University Vienna, Vienna, Austria
- Prof. Dr. Dizdarevic Sabina, Brighton a. Sussex Univ Hospitals NHS Trust (BSUHT), UK
- PD Dr. Ahmadzadehfar Hojjat, Dortmund Klinikum Westfalen, Germany
- Prof. Dr. Mike Sathekge, University of Pretoria and Steve Biko Academic Hospital, SA
- Dr. Milos Petrik, Institute of Molecular and Translational Medicine, Faculty of Medicine and Dentistry, Palacky University
- Prof. Tobias Ross, Department of Nuclear Medicine, Hannover Medical School, Carl- Neuberg-Straße 1, D-30625 Hannover, Germany.
- Dr. Petra Kolenc Peitl, Department of Nuclear Medicine, University Medical Centre Ljubljana, University of Ljubljana, 1000 Ljubljana, Slovenia.
- Prof. Renata Mikolajczak, Radioisotope Centre POLATOM, National Centre for Nuclear Research, 05-400 Otwock, Poland
- Prof. Alicja Hubalewska-Dydejczyk, Department of Endocrinology, Jagiellonian University Medical College, 31-008 Cracow, Poland
- Dr. Jane Sosabowski, Centre for Molecular Oncology, Barts Cancer Institute, Queen Mary University of London
- Dr. Peter Laverman, Radboud University Medical Center Nijmegen, Nijmegen, The Netherlands.
- Prof.Dr. Elzbieta Gumienna-Kontecka, Dept. Of Inorganic Chemistry, University of Wrozlaw, Poland
- Prof. Bernd Pichler, Werner Siemens Imaging Center, Medizinische Universität Tübingen, Tübingen, Germany
![Fig.1: Coronal PET/CT slices of immunocompromised Lewis-rats infected with A. fumigatus in the lung using 68Ga-labelled antifungal “theranostic agents” based on siderophores. Images are showing the lung section of infected (top row) and non-infected (bottom row, control) rats of each compound, respectively. CT images were added to show the severity of the infected lung tissue. Images reproduced from Pfister et al. [1]. Further developments are currently pursued including the use of modified Ferioxamines or the development of artificial siderophores for molecular imaging in close cooperation with H. Haas (Biocenter) and the University of Wrozlaw, inorganic chemistry (E. Gumienna-Kontecka) [2].](https://researchreport.i-med.ac.at/wp-content/uploads/2023/09/Fig.1--768x510.jpg)
![Fig 4: Maximum-intensity projections from static [68Ga] NODAGA-RGD PET scans of a male patient with hepatocellular carcinoma at 13 min (A), 40 min (B), and 76 min (C) after tracer injection. The tracer shows rapid predominant renal elimination with the highest activity in the bladder, kidneys, liver, spleen and intestine. Low background activity is found in the brain, thorax and extremities, resulting in a low radiation dose for the patient. For all three images, the grey-scale is set to the same value. Image from [8]](https://researchreport.i-med.ac.at/wp-content/uploads/2023/09/Fig-4-768x260.jpg)
![Fig. 6: The Era-PERMED TECANT project scheme, with a focus on WP1, probe selection as with substantial contribution from the MUI Department of Nuclear Medicine, reproduced from [11]](https://researchreport.i-med.ac.at/wp-content/uploads/2023/09/Fig.-6-1-768x339.jpg)
![Fig. 8: On the PET-scan prior to therapy local tumour in the prostate bed (red arrow), multiple abdominopelvic and one cervical LN metastases (green arrows) were clearly visible on maximum intensity projection (MIP) (1a) and on fused axial PET/CT-images (1b, 1c). Restaging PET/CT performed eight weeks after administration of 4 cycles of 177Lu-PSMA- 617 with a total accumulated activity of 24.93 GBq showed a markedly reduction of the primary tumour (red arrow) and an impressive partial response of LN-metastases with only small metastases left in the right iliac region (green arrows), as displayed on MIP (2a) and fused axial PET/CT-images (2b, 2c). Image reproduces from [19].](https://researchreport.i-med.ac.at/wp-content/uploads/2023/09/Fig.-8-768x311.jpg)