Anichstraße 35
6020 Innsbruck
Fax: +43 (0)50 504 23055
Email: Christian.Marth@i-med.ac.at
Website: https://frauenheilkunde-innsbruck.tirol-kliniken.at/page.cfm?vpath=index
Research year
Research Branch (ÖSTAT Classification)
301306, 302017, 302022, 302055
Keywords
Biobanking, Biomarker-identification, Clinical studies, Drug testing, Gynaecologic Oncology, Maternal-Fetal Medicine and Obstetric Research, Senology, and Translational Research
Research Focus
- Clinical and preclinical studies in cancers specific to women in terms of prevention, diagnosis, treatment and follow-up care
- Translational cancer research:
- Biomarker identification in breast and gynaecological malignancies
- Specialist interest in increasing the understanding of the development of female cancers
- Drug testing
- Collection of bio-samples (tissues, serum, plasma, ascites…)
- Maternal-Fetal Medicine and Obstetric Research
General Facts
The objective of our Department is to promote and advance research into gynaecological cancers with a particular focus on ovarian and breast cancer concerning prevention, diagnosis, treatment and aftercare. Current trials encompass a range of therapies, including poly(adenosine diphosphate-ribose) polymerase (PARP) or PI3K inhibitors, endocrine therapy, immunotherapy with checkpoint inhibitors, novel cytotoxic substances and antibody-drug conjugates (ADCs), cancer vaccines directed against telomerase and surgical trials.
The majority of clinical trials conducted by the Department are in collaboration with other task forces and research groups, both nationally and internationally. The Department also houses the AGO Studienzentrale, the study office of the non-profit organisation AGO Austria (Austrian Gynaecological Oncology Association).
Preclinical studies are primarily conducted within the Laboratory for Clinical Biochemistry which is headed by Heidi Fiegl. The laboratory is ISO 9001:2015 certified and houses the Department’s biobank, which consists of fresh frozen (FF) tissue, ascites samples (FF tissue biobank) and serum or plasma samples (pre-therapeutic samples and samples from the follow-up period; serum biobank). This biobanking facility has been operational since the 1980s.
Research
Clinical Trials: Gynaecological Oncology
Leader: Christian Marth
A number of gynaecological cancer trials (surgical and therapeutic trials) conducted with the objective of assessing the efficacy and safety of different types of treatment for ovarian (OC), endometrial (EC) and cervical cancer (CC). These treatments encompass targeted therapies, including tumour treatment fields employing insulated transducer arrays, aromatase inhibitors, PI3K inhibitors, ADCs, angiogenesis inhibitors, poly(ADP-ribose) polymerase inhibitors and immunotherapy, particularly checkpoint inhibitors for PD-1 and PD-L1; targeting IL-2R, as well as cancer vaccine directed against telomerase.
Selected trials are described below:
- DUO-O: A Phase III randomised, double-blind, placebo-controlled, multicentre study of durvalumab in combination with chemotherapy and bevacizumab, followed by maintenance durvalumab, bevacizumab and olaparib in newly diagnosed advanced OC.
- MATAO: Maintenance therapy with aromatase inhibitor in epithelial OC: a randomised double-blind placebo-controlled multicentre Phase III trial.
- EPIK-O: A Phase III, multi-centre, randomised (1:1), open-label, active-controlled study to assess the efficacy and safety of alpelisib (BYL719) in combination with olaparib as compared to single agent cytotoxic chemotherapy, in participants with no germline BRCA mutation detected, platinum-resistant or refractory, high-grade (HG) serous OC (HGSOC).
- Ovar 2.29: A randomised Phase III trial of Atezolizumab in combination with bevacizumab and chemotherapy versus bevacizumab and chemotherapy in recurrent OC –.
- LEAP 001: A Phase-III trial comparing immunotherapy (pembrolizumab) and targeted therapy (levatinib) with chemotherapy in patients with stage-III or IV EC or those whose cancer has returned following previous treatment.
- ENGOT-en11: A Phase III, randomised, double-blind study of pembrolizumab versus placebo in combination with adjuvant chemotherapy with or without radiotherapy for the treatment of newly diagnosed high-risk EC after surgery with curative intent.
- ENGOT-cx11: A randomised, Phase III, double-blind study of chemotherapy with or without pembrolizumab for the treatment of high-risk, locally advanced CC.
Leaders: Alexandra Ciresa-König, Andreas Widschwendter:
- PITVIN Follow-Up: Long-term follow-up of patients treated for VIN within the PITVIN trial (Primary Imiquimod Treatment versus Surgery for Vulvar Intraepithelial Neoplasia)
Leader: Irina Tsibulak:
- IVUCCO: International multicentre retrospective study involving 25 institution including patients with unifocal early-stage squamous cell carcinoma of the vulva with >1 mm depth of invasion, who underwent vulvectomy and inguinofemoral lymph node evaluation with current status of surgical margins and pathologic margin distance.
- CONCEPT: Prospective multicentre audit on the value of secondary cytoreductive surgery (SCS) for epithelial OC in the era of PARP-inhibitors.
- MTB project: Retrospective evaluation of oncological outcomes in patients undergoing molecular tumour profiling for gynaecological malignancies
- FREC: Retrospective multicentre analysis of first recurrence of EC.
- SLN in gynaecological malignancies: Evaluation of training and practice patterns in sentinel lymph node mapping for EC and CC.
Leaders: Irina Tsibulak, Verena Wieser
- Pre-ANVU: Feasibility of Groin Ultrasound to Predict Groin Lymph Node Involvement in Patients with Histologically Proven Vulvar Cancer.
- ANVU: A Phase II Randomised Clinical Trial of Ultrasound Groin Monitoring versus Groin Lymph Node Dissection to De-Escalate the Extent of Surgery in Vulvar Cancer.
Clinical Trials: Breast Cancer
Leaders: Christian Marth, Daniel Egle
The breast cancer (BC) care unit successfully participated in several clinical trials in 2023, 2024 and the first quarter of 2025, especially in trials using new targeted therapies such as PARPi (Olaparib in eTNBC), ADCs (i.e. Trastuzumab-Deruxtecan, Sacitucumab-Govitecan, MK2870, Datopotamab Deruxtecan), checkpoint inhibitors (Atezolizumab and Pembrolizumab) and Camizestrant (a next generation oral selective oestrogen receptor degrader).
The following is a concise compilation of the most significant clinical trials:
- ABCSG 45/ PARP-Inhibitor/Olaparib: A prospective, open, randomised, Phase II study of Carboplatin/ Olaparib in the pre-operative treatment of patients with triple-negative primary BC and a positive homologous recombination deficiency (HRD) status.
- DESTINY-BREAST06/Checkpoint Inhibitor/Trastuzumab Deruxtecan: A Phase III randomised, multicentre, open-label study of Trastuzumab Deruxtecan (T-DXd) versus investigator’s choice of chemotherapy in HER2-low, HR+ positive BC patients whose disease has progressed on endocrine therapy in the metastatic setting.
- Trofuse-012: A Phase III randomised, open-label study to compare the efficacy and safety of adjuvant MK-2870 in combination with pembrolizumab (MK-3475) versus treatment of physician’s choice (TPC) in participants with triple-negative BC (TNBC) who received neoadjuvant therapy and did not achieve a pathologically complete response (pCR) with surgery
- TROPION-Breast 04: A Phase III open-label, randomised study of neoadjuvant Datopotamab Deruxtecan (Dato-DXd) plus Durvalumab, followed by adjuvant durvalumab with and without chemotherapy versus neoadjuvant Pembrolizumab plus chemotherapy, followed by adjuvant pembrolizumab with and without chemotherapy for the treatment of adult patients with previously untreated triple-negative or hormone receptor-low/HER2-negative BC.
Leader: Daniel Egle
- ABCSG-52/ATHENE trial: Multicentre open-label, two-arm, randomised single-stage Phase II study of neoadjuvant immunochemotherapy consisting of atezolizumab, trastuzumab, pertuzumab and epirubicin in patients with HER2-positive early-stage BC, focussing on achieving pathological complete remission (pCR).
Leader Christine Brunner:
- CROPSI Study: A randomised, observer-blinded clinical trial evaluating effectiveness of cryotherapy.
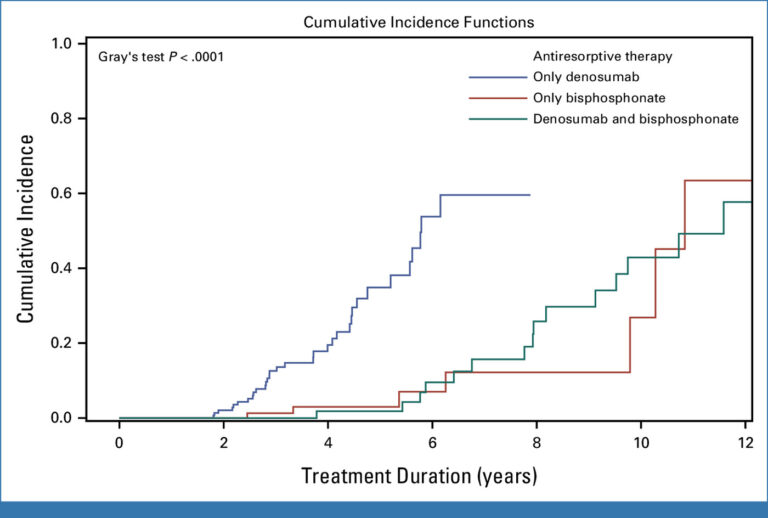
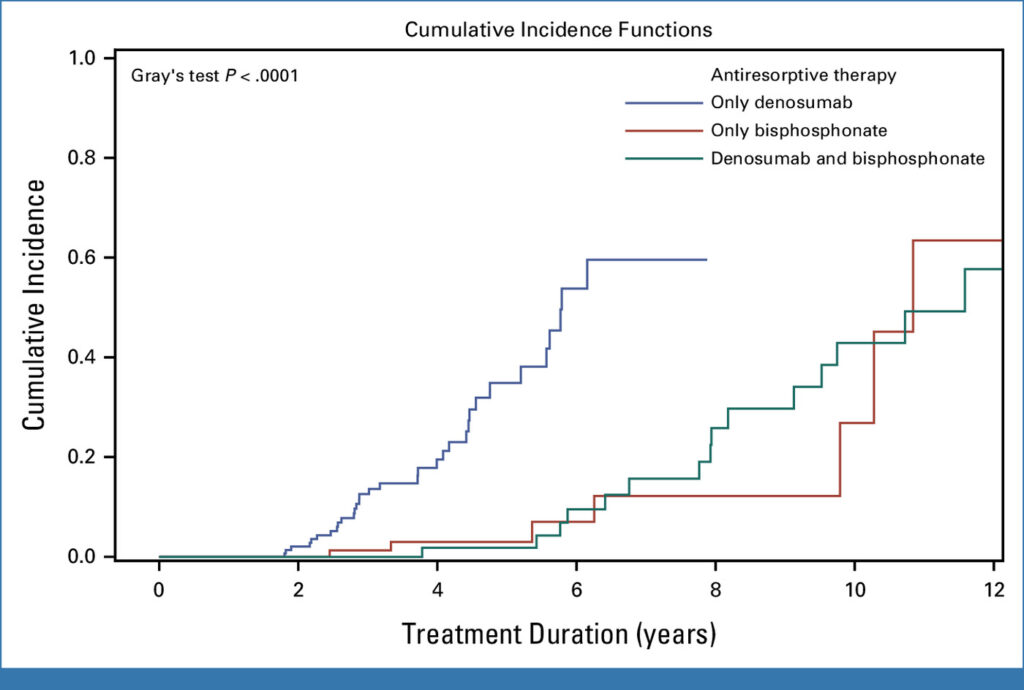
- Incidence of medication-related osteonecrosis of the jaw (MRONJ) in patients with BC: This population-based, multicentre, retrospective observational study used data from all nine breast centres across Tyrol, Austria, between 2000 and 2020. All BC patients receiving anti-resorptive therapy for bone metastases were included as well. The study established that MRONJ is more prevalent in BC patients with bone metastases receiving denosumab than in the general population. Furthermore, patients treated with denosumab also exhibited an earlier onset of MRONJ.. As demonstrated in Figure 1, MRONJ development exhibited substantial temporal variability, contingent on the type of antiresorptive therapy administered (Gray’s test, P < 0.0001).
Clinical trials: Pregnancy
Leader: Samira Abdel Azim
- TRUFFLE2 trial: Perinatal and neurologic 2-year outcome in children with late onset foetal growth restriction- a multicentre randomised controlled trial. The objective of this trial is to ascertain the most effective monitoring methods and thresholds for foetal delivery in cases of late-onset growth restriction, occurring between 32-36 weeks gestation.
Clinical Trials: Urogynaecology
Leader Stephan Kropshofer
- ONIRY: A prospective, non-interventional epidemiological study of the burden of faecal incontinence in women after vaginal delivery.
Translational research
A selection of the most important projects is presented below:
Leader: Daniel Egle
- MESI-STRAT collaboration: The collaboration with Prof. Dr. Thedieck (University of Duisburg-Essen Germany) within the EU project MESI-STRAT is ongoing. MESI-STRAT’s objective was the development of metabolite marker panels measurable in biological fluids. These panels would enable the stratification of BC patients, resistance monitoring and clinical decision-making during endocrine therapy (ET).
Leader: Heidi Fiegl
- BRCA1 DNA methylation in high grade OC (HGOC): In HGOC, the status of homologous recombination deficiency (HRD) is routinely determined to predict how patients will respond to platinum-based therapy or poly (ADP-ribose) polymerase inhibitors (PARPi). In this study, 131 HGOC tissues were tested for epigenetic and genetic aberrations, HRD and expression of BRCA1 mRNA, followed by a survival analysis. The study shows that adding the status of BRCA1 methylation to that of BRCA mutation and HRD status in HGOC is strongly advocated for more accurate prediction.
- Immuno-genomic analyses of HGSOC: In collaboration with Prof. Hubert Hackl (Institute of Bioinformatics, Biocentre), we have proven that genomic instability in HGSOC impacts the tumour’s immunological environment. Tumour-associated macrophages (TAMs) have been demonstrated to play a pivotal role in modulating the immune response. We developed a diagnostic application using RNA sequencing data. This application characterises HGSOC comprehensively and predicts susceptibility and response to combination immunotherapy.
Leader: Daniel Reimer
- Analysis of peritoneal type of tumour spread and HRD status in HGOC: In collaboration with Dr. Simon Schnaiter (Institute of Human Genetics), we showed that the type of tumour spread and the concomitant cytoreduction efficiency is a more effective predictor of survival than HRD. This was in a cohort of HGOC patients who had predominantly not received PARPi. It has been hypothesised that HRD may be an incidental surrogate marker for tumour spread and associated cytoreduction efficiency. Further research is needed to determine whether this also applies to sensitivity to PARPi.
Leader: Verena Wieser
- Tumoral programmed cell death 1 (PD1) expression in EC was analysed in cancerous and non-cancerous endometrial tissues and The Cancer Genome Atlas (TCGA) data. Tumoral RNA expression of genes controlling the immune checkpoint, programmed cell death 1 (PD1, encoded by PDCD1), its ligand (PDL1, encoded by CD274), and interferon gamma (IDNG) was also analysed. POLE-mutated and mismatch repair-deficient (MMRd) tumours showed the highest expression of PD-1 and Increased expression of these markers was linked to improved prospects of survival. Evaluation of these genes could be the key to accurately stratifying patients based on their suitability for immune checkpoint inhibitors.
Leaders: Alain Zeimet, Katharina Steger
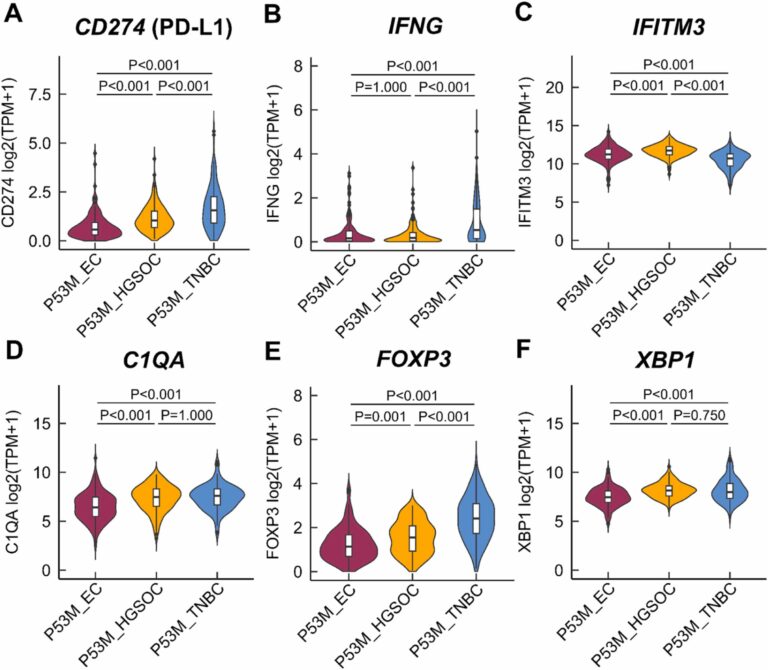
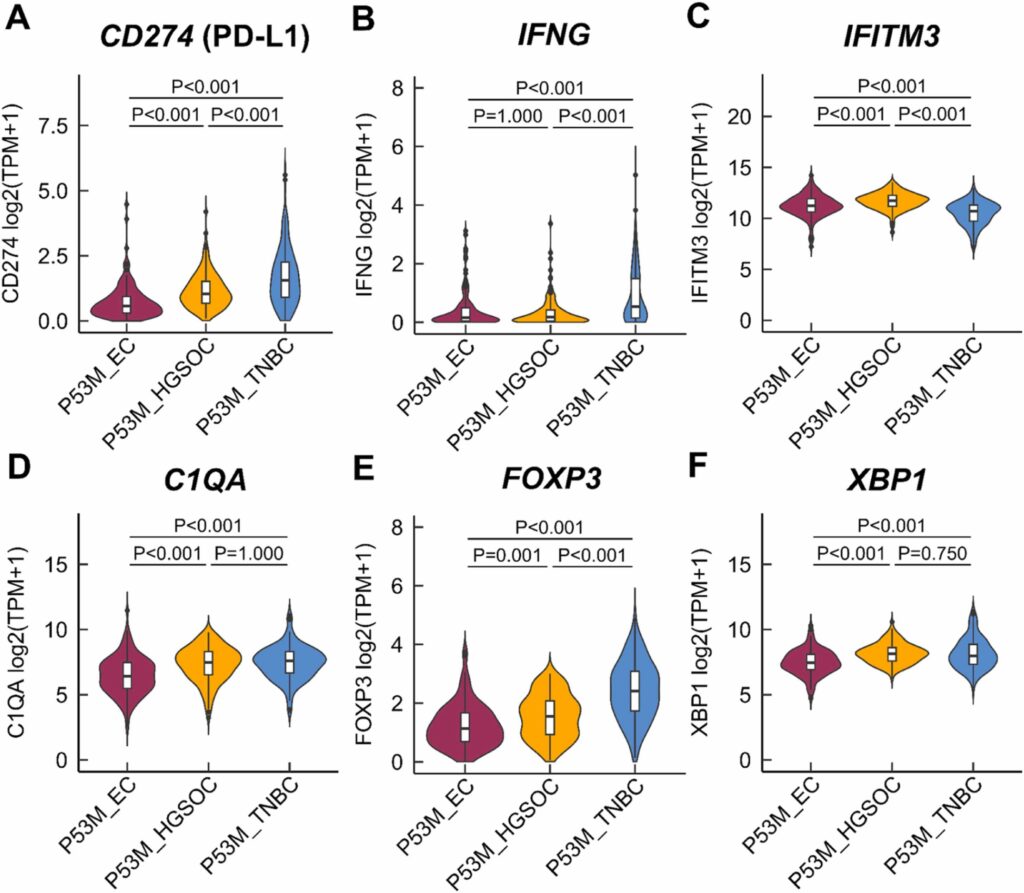
- Differences in immunogenicity of TP53-mutated (TP53mut) cancers with low tumour mutational burden (TMB): This study investigated why TP53mut EC was the only tumour in large randomised Phase III clinical trials to show survival benefit from immune checkpoint inhibitors (ICIs) added to chemotherapy compared to other TP53mut cancers with low TMB, such as HGSOC and triple-negative BC (TNBC). The immune contexture differed significantly between the three tumour entities. The presence of a higher proportion of regulatory T-cells (Tregs) and M2-like macrophages (TAMs), along with elevated levels of C1QA, FOXP3 and XBP1, indicates a more immunosuppressive microenvironment in TP53mut HGSOC and TNBC compared to TP53mut EC.
Maternal-Fetal Medicine and Obstetric Research
Leader: Samira Abdel-Azim
- Placental histopathologic changes in maternal cardio-metabolic conditions – a retrospective study
Leader: Irene Mutz-Dehbalaie
- Myocardial performance-Index (MPI) in diabetic pregnancies und short term neonatal outcome – a retrospective study
Pictures
Selected Publications
- Christian, Marth; Richard G, Moore; Mariusz Bidziński; Sandro,Pignata; Ali, Ayhan; M Jesús, Rubio; Mario, Beiner; Marcia, Hall; Christof, Vulsteke; Elena Ioana, Braicu; Kenzo, Sonoda; Xiaohua Wu; Sophia, Frentzas; André, Mattar; Stephanie, Lheureux; Xiaojun, Chen; Kosei, Hasegawa; Manuel, Magallanes-Maciel; Chel, Hun, Choi; Mariia, Shalkova; Diego, Kaen; Peng-Hui, Wang; Regina, Berger; Chinyere E, Okpara; Jodi, McKenzie; Lili, Yao; Robert, Orlowski; Vivek, Khemka; Lucy, Gilbert; Vicky, Makker; ENGOT-en9/LEAP-001 Investigators.: First-Line Lenvatinib Plus Pembrolizumab Versus Chemotherapy for Advanced Endometrial Cancer: A Randomized; Open-Label; Phase III Trial. JOURNAL OF CLINICAL ONCOLOGY. 2024; 26:JCO240326.
PMID: 395955 doi: 0.200/JCO-24-0326.
- Colombo, Nicoletta; Biagioli, Elena; Harano, Kenichi; Galli, Francesca; Hudson, Emma; Antill, Yoland; Choi, Chel Hun; Rabaglio, Manuela; Marme, Frederic; Marth, Christian; Parma, Gabriella; Farinas-Madrid, Lorena; Nishio, Shin; Allan, Karen; Lee, Yeh Chen; Piovano, Elisa; Pardo, Beatriz; Nakagawa, Satoshi; Mcqueen, John; Zamagni, Claudio; Manso, Luis; Takehara, Kazuhiro; Tasca, Giulia; Ferrero, Annamaria; Tognon, Germana; Lissoni, Andrea Alberto; Petrella, Mariacristina; Laudani, Maria Elena; Rulli, Eliana; Uggeri, Sara; Ginesta, M. Pilar Barretina; AtTEnd Study Grp: Atezolizumab and chemotherapy for advanced or recurrent endometrial cancer (AtTEnd): a randomised, double-blind, placebo-controlled, phase 3 trial. LANCET ONCOLOGY. 2024; 25(9); 1135-1146.
PMID: 39102832 doi: 10.1016/S1470-2045(24)00334-6.
- Dugo, Matteo; Huang, Chiun-Sheng; Egle, Daniel; Bermejo, Begona; Zamagni, Claudio; Seitz, Robert S.; Nielsen, Tyler J.; Thill, Marc; Anton-Torres, Antonio; Russo, Stefania; Ciruelos, Eva Maria; Schweitzer, Brock L.; Ross, Douglas T.; Galbardi, Barbara; Greil, Richard; Semiglazov, Vladimir; Gyorffy, Balazs; Colleoni, Marco; Kelly, Catherine M.; Mariani, Gabriella; Del Mastro, Lucia; Blasi, Olivia; Callari, Maurizio; Pusztai, Lajos; Valagussa, Pinuccia; Viale, Giuseppe; Gianni, Luca; Bianchini, Giampaolo: The Immune-Related 27-Gene Signature DetermaIO Predicts Response to Neoadjuvant Atezolizumab plus Chemotherapy in Triple-Negative Breast Cancer. CLINICAL CANCER RESEARCH. 2024; 30(21); 4900-4909.
PMID: 39308141 doi: 10.1158/1078-0432.
- Colleselli-Tuertscher, Valeria; Hafenmayr, Marina; Ciresa-Koenig, Alexandra; Trinker, Michael; Maier, Sarah; Toth, Bettina; Seeber, Beata: Retrospective cohort study comparing success of medical management of early pregnancy loss in pregnancies conceived with and without medical assistance. FERTILITY AND STERILITY. 2024; 121(5); 824-831.
PMID: 38211763 doi: 10.1016/j.fertnstert.2024.01.011. - Gabriel, Rinnerthaler; Daniel, Egle; Rupert, Bartsch; Clemens A,, Schmitt; Andreas, Petzer; Marija Balic; Edgar, Petru; Ursula,Denison; Christian F, Singer; Vesna, Bjelic-Radisic; Simon, Peter Gampenrieder; Michael, Knauer; Karl, Sotlar; Christine, Brunner; Florian, Posch; Dominik, Hlauschek; Lidija, Sölkner; Zsuzsanna, Bago-Horvath; Martin, Filipits; Manuela, Gili; Magdalena, Ritter; Verena, Wieser; Carmen, Albertini; Nadja, Zaborsky; Lukas, Weiss; Maximilian, Marhold; Bruno, Schneeweiss; Renate, Pusch; Michael Gnant; Richard Greil.: Neoadjuvant atezolizumab in combination with dual HER2 blockade plus epirubicin in women with early HER2-positive breast cancer: the randomized phase 2 ABCSG-52/ATHENE trial. NATURE CANCER. 2025; 6(1): 41-50.
PMID: 3982025 doi: 10.1038/s43018-024-00890-2. - Christine, Brunner; Marjan, Arvandi; Christian, Marth; Daniel, Egle; Florentina, Baumgart ; Miriam, Emmelheinz; Benjamin, Walch; Johanna, Lercher; Claudia, Iannetti; Ewald, Wöll ; Agnes, Pechlaner; August, Zabernigg; Birgit, Volgger; Maria, Castellan; Oliver, Tibor Andraschofsky; Alice, Markl ; Michael, Hubalek; Michael, Schnallinger; Sibylle, Puntscher; Uwe, Siebert; Sebastian, Schönherr; Lukas, Forer; Emanuel, Bruckmoser; Johannes, Laimer.: Incidence of Medication-Related Osteonecrosis of the Jaw in Patients With Breast Cancer During a 20-Year Follow-Up: A Population-Based Multicentre Retrospective Study. JOURNAL OF CLINICAL ONCOLOGY. 2025; 43(2); 80-88.
PMID: 396356 doi: 0.200/JCO.24.007. - Katharina, Steger; Heidelinde, Fiegl; Barin, Feroz; Katharina, Leitner; Christian, Marth; Hubert, Hackl; Alain Gustave, Zeimet.: Differences in immunogenicity of TP53-mutated cancers with low tumor mutational burden (TMB) A study on TP53mut endometrial-; ovarian- and triple-negative breast cancer. EUROPEAN JOURNAL OF CANCER. 2025; 29: 5320.
PMID: 39986022 doi: 10.1016/j.ejca.2025.115320
Selection of Funding
Clinical Trials:
- ABCSG 60 – CAMBRIA 1,
- ABCSG 62 – CAMBRIA 2,
- NIS DESTINY BREAST RESPOND,
- TRUFFLE-2-Studie
Collaborations
Collaborations in Clinical Trials with (inter)national research groups:
- AGO Austria – Arbeitsgemeinschaft Gynäkologische Onkologie
- ABCSG – Austrian Breast & Colorectal Cancer Study Group
- ENGOT – European Network for Gynaecological Oncological Trial groups
- GCIG – Gynaecologic Cancer InterGroup (Cooperation with 25 international trial groups)
International collaboration with trial groups outside of ENGOT, GCIG or ABCSG trials:
- Dr. Jalid Sehouli, Charité Berlin, Germany
- EORTC – European Organisation for Research and Treatment of Cancer
- Prof. Christina Fotopoulou, Imperial College London, UK
- Dimitrios Nasioudis and Prof. Rob Giuntoli, University of Pennsylvania, Abramson Cancer Centre, USA
- Nicolò Bizzarri, Gynecologic Oncology Unit, Catholic University of the Sacred Heart, Policlinico Agostino Gemelli IRCCS, Italy
Collaborations in Translational research projects:
- Dr. Kathrin Thedieck, Dept. of Biochemistry, University of Duisburg-Essen, Essen, Germany
- Dr. Robert Zeillinger, Medical University Vienna, Vienna, Austria
- Dr. Tim Fenton, Institute for Life Sciences, University of Southampton, Southampton, United Kingdom