Müllerstrasse 59
6020 Innsbruck
Email: lars.klimaschewski@i-med.ac.at
Website: https://www.i-med.ac.at/ahe/histologie-embryologie/
Research year
Research Branch (ÖSTAT Classification)
106049 Ultrastrukturforschung 106052 Zellbiologie 301107 Histologie 301114 Zellbiologie 301304 Medizinische Biologie
Keywords
autophagy, cellular electron microscopy, endosomal/lysosomal pathways, membrane trafficking, and organelle ultrastructure
Research Focus
High-resolution microscopy with emphasis on ultrastructural analyses of subcellular architecture in the context of intact tissues and cells from model organisms and patient samples
General Facts
The Institute of Histology and Embryology at the Medical University of Innsbruck provides lecture series and practical courses in Histology and Microscopic Anatomy for undergraduate students in Human Medicine and Molecular Medicine. In addition, we offer special lectures and courses on sub-microscopic morphology and advanced cellular electron microscopy for MD and PhD students. Research of the Cellular Electron Microscopy group focuses on ultrastructural aspects of intracellular membrane trafficking and signalling, performed in close collaboration with local and international research groups.
Research
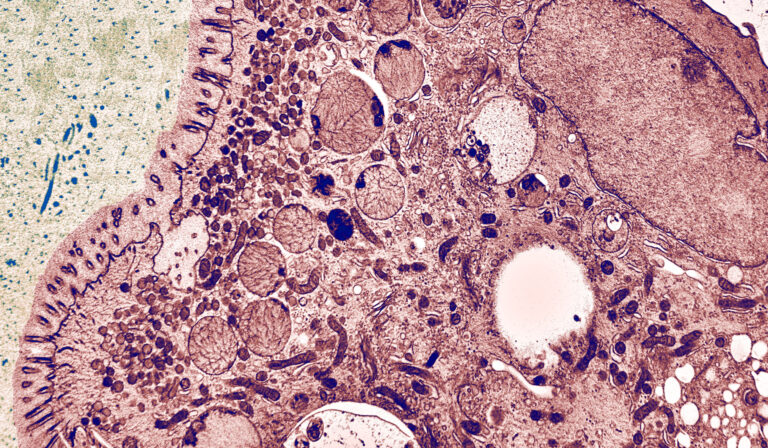
The Cellular Electron Microscopy Group of M. W. Hess employs high-resolution microscopy for the ultrastructural analysis of subcellular architecture in the context of intact tissues and cells from model organisms and patient samples. Major focus of our cell biological studies is intracellular membrane trafficking and signalling in health and disease (performed in collaboration with the groups of L. A. Huber and D. Teis: Division of Cell Biology, T. Müller, A. Janecke, G.F. Vogel: Department of Paediatrics I), as well as the structural basis of bio-adhesion (together with P. Ladurner and W. Salvenmoser: Department of Zoology, Univ. Innsbruck). Advanced cryo-based immuno-electron microscopy and electron tomographic 3D-reconstruction are our methods of choice. We investigate (genome-edited) human and animal cell models (Perez Carion et al. 2018, Frontiers in molecular neuroscience 11:64; Yordanov et al. 2019, Traffic, 20:674), biopsy samples from patients (Vogel et al 2017, Traffic 18: 453) and eukaryotic model organisms such as mice, yeast (Adell et al. 2017, Elife 6:e31652), flatworms (Pjeta et al. 2019, Philosophical Transactions of the Royal Society B 374:20190194) and Hydra (Rodrigues et al. 2016, BMC Zoology 1:3). Membrane trafficking is studied with special emphasis on the biogenesis and maturation of endocytic compartments, cargo biosynthesis and recycling, as well as autophagy. Among others, we are also interested in the relationships between cargo biosynthesis and trafficking, cytoskeletal architecture and maintenance of cellular polarity. All these processes are severely disturbed in congenital gastrointestinal disorders. Microvillus Inclusion Disease (MVID), for example, is a rare, fatal hereditary disease that affects infants soon after birth (Figure 1).
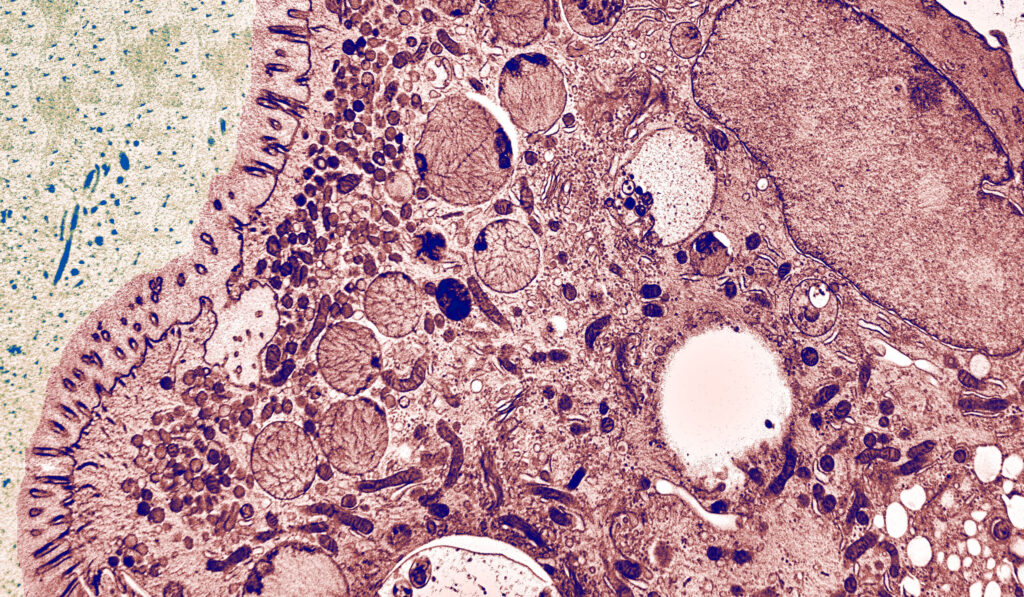
The clinical appearance presents severe watery, non-inflammatory diarrhoea, nutrient malabsorption and metabolic acidosis. In general, MVID patients depend on total parenteral nutrition. Small bowel transplantation is the only curative therapy, but many patients die within the first few years of life. In previous years, our multidisciplinary cell biological-clinical team provided mechanistic insight into the pathophysiology of MVID by identifying mutations in several proteins to be causative for the disorder (i.e., motor protein Myosin5b, or the apical membrane protein Syntaxin3, or its interaction partner Syntaxin-binding protein 2, or Unc-45 Myosin Chaperone A). Those mutations disrupt selective apical cargo trafficking and exocytosis in epithelial absorptive cells of the small intestinum, leading to mislocalisation of pivotal brush border ion transporters relevant for physiological enterocyte function (T Müller et al. 2008, Nature genetics 40:1163; Wiegerinck et al. 2014, Gastroenterology 147:65; Vogel et al. 2017, J Clin Invest-Insight 2(14): e94564; Vogel et al 2017, Traffic 18: 453; Duclaux-Loras et al 2022, J Clin Invest 132(10):e154997). Ultimately, we perform methodological research for precise localisation of macromolecules in the context of natively cryo-immobilised cells.
Pictures
Selected Publications
Hess, Michael W.; Krainer, Iris M.; Filipek, Przemyslaw A.; Witting, Barbara; Gutleben, Karin; Vietor, Ilja; Zoller, Heinz; Aldrian, Denise; Sturm, Ekkehard; Goldenring, James R.; Janecke, Andreas R.; Mueller, Thomas; Huber, Lukas A.; Vogel, Georg F.: Advanced Microscopy for Liver and Gut Ultrastructural Pathology in Patients with MVID and PFIC Caused by MYO5B Mutations. JOURNAL OF CLINICAL MEDICINE. 2021; 10(9); 1901. PubMed: 33924896 doi: 10.3390/jcm10091901
Bertemes, Philip; Pjeta, Robert; Wunderer, Julia; Grosbusch, Alexandra L.; Lengerer, Birgit; Gruener, Kevin; Knapp, Magdalena; Mertens, Birte; Andresen, Nikolas; Hess, Michael W.; Tomaiuolo, Sara; Zankel, Armin; Holzer, Patrik; Salvenmoser, Willi; Egger, Bernhard; Ladurner, Peter: (Un)expected Similarity of the Temporary Adhesive Systems of Marine, Brackish, and Freshwater Flatworms. INTERNATIONAL JOURNAL OF MOLECULAR SCIENCES. 2021; 22(22); 12228. PubMed: 34830109 doi: 10.3390/ijms222212228
Hess, Michael W.; Huber, Lukas A.: Measuring lysosomal size and frequency by electron microscopy. METHODS IN CELL BIOLOGY. 2021; 164(S); 47-61. PubMed: 34225918 doi: 10.1016/bs.mcb.2020.10.019
Duclaux-Loras, Remi; Lebreton, Corinne; Berthelet, Jeremy; Charbit-Henrion, Fabienne; Nicolle, Ophelie; de Courtils, Celine Revenu; Waich, Stephanie; Valovka, Taras; Khiat, Anis; Rabant, Marion; Racine, Caroline; Guerrera, Ida Chiara; Baptista, Julia; Mahe, Maxime M.; Hess, Michael W.; Durel, Beatrice; Lefort, Nathalie; Banal, Celine; Parisot, Melanie; Talbotec, Cecile; Lacaille, Florence; Ecochard-Dugelay, Emmanuelle; Demir, Arzu Meltem; Vogel, Georg F.; Faivre, Laurence; Rodrigues, Astor; Fowler, Darren; Janecke, Andreas R.; Mueller, Thomas; Huber, Lukas A.; Rodrigues-Lima, Fernando; Ruemmele, Frank M.; Uhlig, Holm H.; Bene, Filippo Del; Michaux, Gregoire; Cerf-Bensussan, Nadine; Parlato, Marianna: UNC45A deficiency causes microvillus inclusion disease-like phenotype by impairing myosin VB-dependent apical trafficking. JOURNAL OF CLINICAL INVESTIGATION. 2022; 132(10); e154997. PubMed: 35575086 doi: 10.1172/JCI154997
Selection of Funding
Collaborations
Peter Ladurner, Bert Hobmayer (Department of Zoology, University of Innsbruck, Austria), James R. Goldenring (Section of Surgical Sciences, Epithelial Biology Center, and Department of Cell & Developmental Biology, Vanderbilt University School of Medicine, Nashville, TN, USA)