Schöpfstraße 41
6020 Innsbruck
Fax: +43 512 900373700
Email: hygiene-bakteriologie@i-med.ac.at
Website: https://www.i-med.ac.at/hygiene/_
Research Branch (ÖSTAT Classification)
303026, 303002, 303015, 303013, 303020
Keywords
antifungal resistance, complement, dendritic cells, fungal pathogens, hygiene, immunity, infectious diseases, mycoses, N-chlorotaurine, and nosocomial infections
Research Focus
Understanding infections: from pathogenesis to diagnosis
- Tasks comprise research, teaching and laboratory diagnosis of infectious diseases, hospital and technical hygiene.
- Scientific activities cover fungal pathogenicity, antifungal resistance, virulence factors, host pathogen-interactions, basic immunological research, fungal carcinogenesis, antimicrobial agents and prevention of nosocomial infections.
- HMM seeks to prevent illness and death from targeted infectious disease threats (Figure 1).
General Facts
Infectious diseases are becoming one of the most frequent causes of death world-wide. Understanding the biological principles underlying the mechanisms by which infectious agents adapt and undermine the defence mechanisms of a host is critical for fighting diseases. HMM conducts basic and translational research into molecular mechanisms of pathogenesis of bacterial, viral or fungal infections and strategies for their prophylaxis and therapy. HMM’s mission is the coordination and strategic alignment of translational infection research with the aim of developing new diagnostic, preventative and therapeutic methods for the treatment of infectious diseases. To achieve this, HMM has formed thematic translational units of scientists, each dedicated to one specific pathogen or infectious disease. HMM is one of the largest microbiology diagnostic laboratories in Austria, with an average sample throughput of 250 000 specimens per year. HMM is associated with all major hospitals in Tyrol, seeking it in a key position in the diagnostic laboratory landscape in Austria. The working groups Reinhard Würzner, Doris Wilflingseder and Wilfried Posch cover the topic “Exploiting immune response”, the working groups Michaela Lackner and Cornelia Lass-Flörl with Cornelia Speth and Ulrike Binder investigate fungal infections. “N-chlorotaurin” is the main research focus of Markus Nagl. The mission of HMM is to bridge the gap between basic and translational research into microbial pathogenesis.
Research
Understanding fungal infections
Cornelia Lass-Flörl, Ulrike Binder
In 2015, a “Christian-Doppler-Laboratory for Invasive Fungal Infections” was established. Approximately 600 of an estimated 2 million fungal species on earth cause diseases in humans, the most important of which are Candida, Aspergillus, Mucorales and Cryptococcus. Fungal infections are increasing and are associated with excessive morbidity and mortality. Over 300 million people are acutely or chronically infected, leading to death, long term illness and reduced working capacity. The reasons for an emergence are likely multifactorial, e.g. the advent of medical progress, the successful application of immunosuppression in transplanted patients and the use of immunomodulatory agents for the treatment of various diseases from cancer to rheumatoid arthritis.
CD-Fungus attempts to unravel scientific questions raised by implementing 3 modules, which will ultimately advance our understanding of fungal pathology, improve diagnosis and treatment of mucormycosis and enhance patients’ outcome and safety in terms of prevention of nosocomial and hospital-associated infections. The laboratory was closed in 2022.
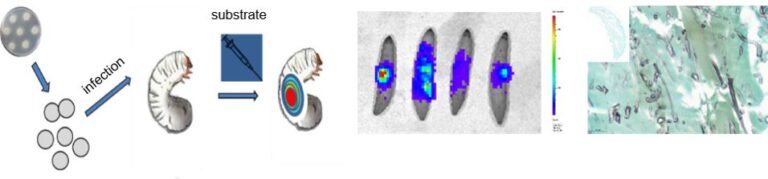
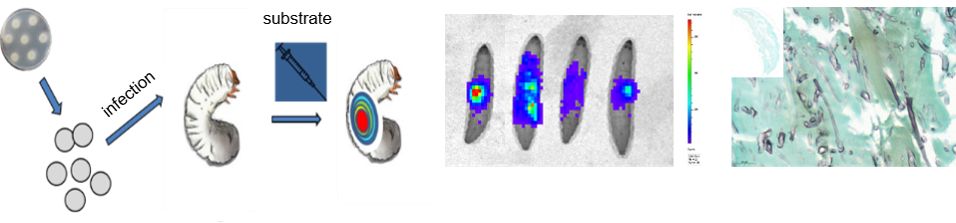
Understanding the pathogen and why it has the ability to cause infection is one key element to combat disease. To address questions such as the role of certain genes for virulence or test the efficacy of novel antifungal drugs, alternatives in vivo models are needed. In recent years, the alternative infection model Galleria mellonella was extensively used and optimised to address studies of fungal pathogenicity, the establishment of disease and the efficacy of new antifungal drugs. Here, a special focus has been placed on Mucorales as in this group of fungi factors and molecular mechanisms that confer virulence, immune evasion or antifungal efficacy are still insufficiently know. With the generation of bioluminescent reporter strains and the simultaneously deletion of genes potentially necessary for virulence, another tool is available to us, which we currently use to investigate onset of infection and efficacy of antifungal drugs. As resistance is an emerging problem in pathogenic fungi, plus currently used drug exhibit very high toxicity in human, new and better drugs are urgently needed. Currently, modified antifungal agents are screened by the use of alternative test systems to select promising candidates for further analysis. In other projects, the luciferase system is currently employed as an in vivo tool to monitor gene transcription.
Fungal Carcinogenesis
Cornelia Speth
In collaboration with the departments of Surgery and Pathology, the Speth’s group placed the research focus on three aspects of fungus-associated carcinogenesis: (1) Detection and identification of fungi in a selected spectrum of tumour types; for this purpose, a bunch of different methods is established and combined to verify this exciting possibility. (2) Detailed investigation of the Malassezia-complement interaction in order to understand precisely the mechanism how fungi and fungus-induced inflammation can contribute to pancreatic cancer; this knowledge of the molecular processes is the prerequisite for an immune-based therapy that targets the excessive complement activation. (3) Study of the possibility to eradicate the fungus from the dense desmoplastic tumour microenvironment of PDAC by using T-cells with chimeric antigen receptors against fungal chitin; in close collaboration with Prof. Abken from the Leibniz Institute of Immunotherapy, the antifungal efficacy of these CAR-T cells is tested for Malassezia as well as other fungal species that may contribute to carcinogenesis.
Understanding the molecular mechanisms of antifungal resistance provide novel avenues for drug discovery
Michaela Lackner
Our strategy is to decipher the molecular basis of antifungal resistance and to generate structure- and function-based information for drug discovery and development.
We focus specifically on azole drugs, which target the cytochrome P450 enzyme lanosterol 14α-demethylase (LDM), a key enzyme in the fungus-specific ergosterol biosynthesis pathway. Azoles binding to the ligand-binding pocket (LBP) result in a depletion of ergosterol, accumulation of toxic and subsequent reduced membrane fluidity and cell integrity. Main resistance mechanisms are upregulation of drug efflux pumps, overexpression of LDMs and modifications in the LBP of LDMs.
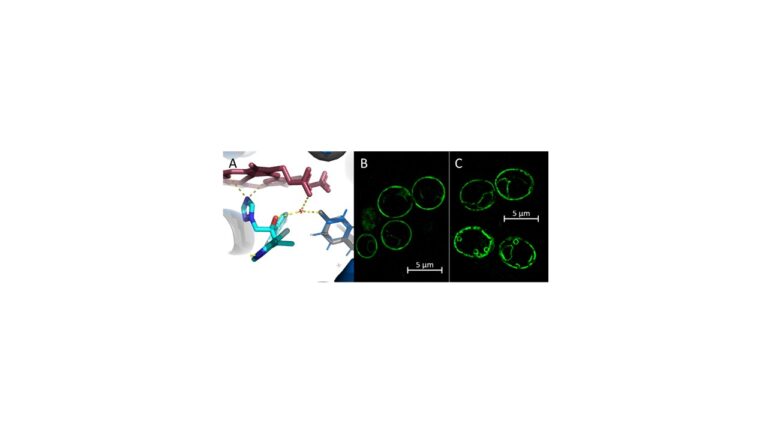
Our research focuses on measuring the impact and elucidating the structural basis of key AA substitutions in the LBP of LDMs on azole resistance and the characterisation of drug efflux pumps and their substrate preference. AA substitutions in the LBP of LDMs can lead to a loss of key water-mediated hydrogen bond networks between the ligand and the LBP (Figure x A).
The triazole structure determines LDM binding capacities and proneness for drug efflux. In sum, these factors affect drug efficacy, azole potency and liver toxicity.
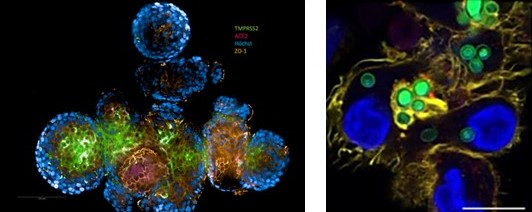
We use Saccharomyces cerevisiae as a heterologous overexpression model to characterise and elucidate LDMs and drug efflux pumps of human-pathogenic fungi. GFP-labels proteins allow studying protein trafficking and localisation via super resolution fluorescence microscopy (Figure x B and C).
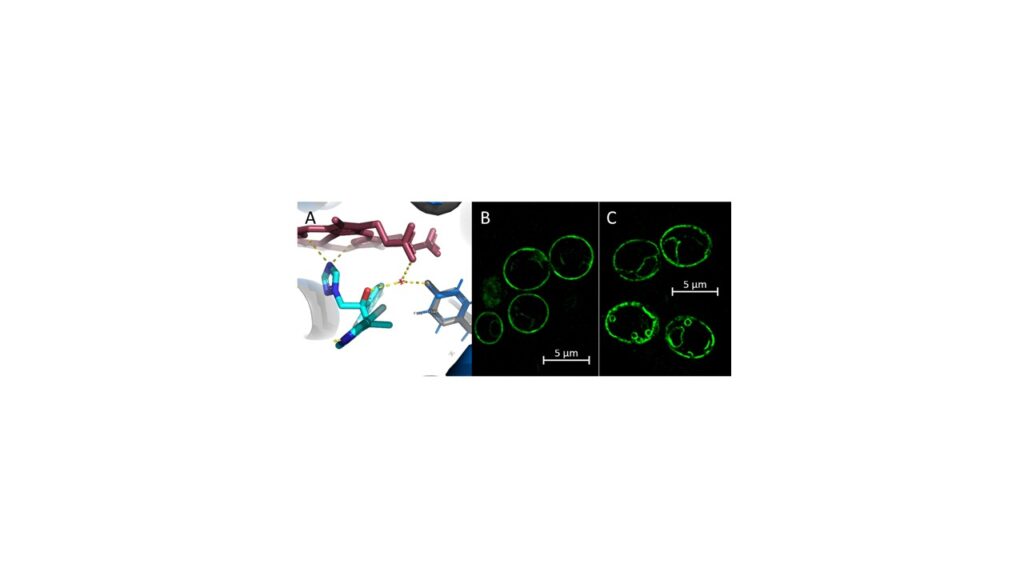
Enzyme kinetic assays and LDM crystal structures offer the opportunity for function- and structure-guided development of a new generation of more effective, broad-spectrum azole drugs that have stronger binding affinities and improved specificity. Our aims are (a) to discover and characterise drug resistance mechanisms and (b) to establish a comprehensive practical tool for the screening and discovery of novel broad-spectrum antifungals.
Exploiting Immune Response
Reinhard Würzner
Complement is connecting a variety of immunities and also represents a tightly controlled area of the innate immune system, which facilitates phagocytosis and the killing of invading pathogens. Factor H (FH) is the main fluid-phase inhibitor of the alternative pathway. Many pathogens interact with complement to evade the immune system of the host; some hijack FH from the host and protect themselves from complement-dependent killing. The group of Reinhard Würzner has examined the influence of glucose on FH-binding. They also continued their work on the characterisation and detection of various shigatoxin forms and their association with haemolytic uraemic syndrome. In the COVID 19 pandemics, the role of complement and the development of avidity in the time course of infection was studied: interestingly there was a marked increase in avidity of SARS-CoV-2 antibodies 7-8 months after infection which was, surprisingly, not diminished in old age; the avidity itself was highly dependent on the vaccines used and – unexpectedly – better when a vector-based vaccine was used initially.
Crosstalk in infection in disease-on-chip models
Doris Wilflingseder
Another focus is on antigen-presenting cells (i.e. dendritic cells (DCs), macrophages), innate humoral components (i.e. complement) and their interaction with pathogens at barrier sites. Thus, the Wilflingseder group is studying entry mechanisms of various pathogens (HIV-1 vs. HIV-2, coronaviruses, Aspergillus spp., co-infection models) to unravel the effect of tissues or the opsonisation pattern of pathogens on inflammatory or de-regulated immune processes. As illustrated, opsonisation has a severe impact on DC function in terms of maturation, anti-viral immunity and T cell stimulation as well as activation of restriction factors. Further, the role of complement in metabolic reprogramming of dendritic cells during viral infections is currently studied as part of the FWF-funded “Cellular Basis of Diseases – CBD” PhD graduate programme (https://phd-cbdiseases.i-med.ac.at/). Immune cell crosstalk with tissues will be assessed within the research training network ´CONNECT´ funded in December 2022 by the MUI and coordinated by Doris Wilflingseder. During the last decade, the Wilflingseder group developed, optimised and standardised highly differentiated human cell culture models, in order to allow research into the initial interactions between various pathogens in the acute phase of infection. The complex in vitro systems of lung, intestine and other mucosal origin allow integration of various immune cells and therefore enable detailed analyses close to the in vivo situation. 3D barrier models, differentiated apical-out organoids for high content screening as well as interconnected micro-physiological systems (MPS), are applied in further studies to investigate infection, inflammation and tissue damage as well as to identify novel therapeutic targets.
Humoral and Cellular Immunity mediated by emerging infectious diseases
Wilfried Posch
Since the beginning of the COVID-19 pandemic, numerous variants of this novel coronavirus, SARS-CoV‑2, have emerged causing an immense burden of mortality and morbidity among the global population. In the beginning of the pandemic, the Posch group investigated immunity induced in COVID-19 patients in mild, severe and critical disease progression (Lafon E et al Front Immunol. 2021). With the development and approval of COVID-19 vaccines, additional studies were performed to investigate not only humoral, but also cellular immunity induced by these vaccines after first, second and booster doses. A specific interest during these studies was the neutralisation ability against SARS-CoV-2 variants of concern of the various vaccination cohorts or in convalescent individuals. Moreover, the anti-viral immunity induced in young and elderly individuals was studied. Due to the increasing numbers of breakthrough infections by upcoming Omicron sub-variants, a closer look was placed on mucosal immunity in vaccinated and convalescent individuals, and differences were found in neutralisation and antibody titres depending on the immunisation regimen. For these analyses as well as for testing potential new COVID-19 drugs, the Posch and the Wilflingseder group joined forces and thus, analyses also in a personalised approach developed by the Posch group were tested in the standardised respiratory 3D model. Further, in collaboration, antimicrobial efficacy and inactivation kinetics of a novel LED based UV-irradiation technology as an alternative decontamination method was analysed. During the last three years, the Posch group established a well-functioning translational network, allowing to immediately establish, standardise and optimise tools for studying emerging infections, which is reflected in the high output of the junior faculty. As faculty member of the CONNECT graduate program, Wilfried Posch further evaluates SARS-CoV-2 interactions at barrier sites within an immune-competent model.
N-chlorotaurine
Markus Nagl
N-chlorotaurine (NCT), a long-lived oxidant produced by activated human leukocytes, has been synthesised as sodium salt in our laboratory and is being investigated as a new antiseptic for the topical treatment of infections in multiple, including sensitive body regions.
Basic research currently comprises its microbicidal activity against viruses including SARS-CoV-2, and its microbicidal activity against planktonic forms and biofilms of bacteria in the field of surgery and dentistry. Latest in-vivo investigations cover the bronchopulmonary system (efficacy of inhaled NCT in mouse fungal pneumonia, confirmatory phase II inhalation of NCT in viral infections planned) as well as various other topical applications, such as the skin and mucous membranes, the ear-nose-throat region, the urinary tract, the eye and organ abscesses. Advantages of NCT include high tolerability, a broad spectrum of activity against all strains of pathogens without inducing resistance, inactivation of virulence factors of pathogens, enhancement of microbicidal activity in the presence of body fluids and anti-inflammatory effects.
Laboratory Diagnostics, Hospital and Technical Hygiene
The HMM division fulfils its tasks in the detection and identification of pathogens that cause infections. These cover bacteriology, parasitology, mycobacteriology and mycology. The diagnostic laboratories are certified according to ISO 9001:2009. Special parts are controlled by external audits in accordance with §67 Austrian Medicines Law and FDA, Manufacturing and Product Quality division. Within the sector of hospital and technical hygiene (accredited under ISO/IEC 17025 and ISO/IEC 17020), guidelines for the prevention of infectious diseases are developed and controlled in line with the statutory requirements for technical facilities (e.g. disinfection machines).
Pictures
Selected Publications
Bellotti R, Speth C, Adolph TE, Lass-Flörl C, Effenberger M, Öfner D, Maglione M (2021)
Micro- and Mycobiota Dysbiosis in Pancreatic Ductal Adenocarcinoma Development
Cancers (Basel). 2021 Jul 8;13(14):3431. doi: 10.3390/cancers13143431. PMID: 34298645
Toepfer S, Lackner M, Keniya MV, Monk BC. Functional Expression of Recombinant Candida auris Proteins in Saccharomyces cerevisiae Enables Azole Susceptibility Evaluation and Drug Discovery. J Fungi (Basel). 2023 Jan 27;9(2):168. doi: 10.3390/jof9020168. PMID: 36836283
Harpf V, Kenno S, Rambach G, Fleischer V, Parth N, Weichenberger CX, Garred P, Huber S, Lass-Flörl C, Speth C, Würzner R. Influence of Glucose on Candida albicans and the Relevance of the Complement FH-Binding Molecule Hgt1 in a Murine Model of Candidiasis. Antibiotics. 2022 Feb 16;11(2):257. doi: 10.3390/antibiotics11020257. PMID: 35203859
Posch W, Vosper J, Noureen A, Zaderer V, Witting Ch, Bertacchi G, Gstir R, Filipek PA, Bonn GK, Huber LA, Bellmann-Weiler R, Lass-Flörl C, Wilflingseder D. C5aR inhibitio of nonimmune cells suppresses inflammation and maintains epithelial integrity in SARS-CoV-2-infected primary human airway epithelia. J Allergy Clin Immunol. 2021 Jun;147(6):2083-2097. PMID: 33852936
Lafon E, Jäger M, Bauer A, Reindl M, Bellmann-Weiler R, Wilflingseder D, Lass-Flörl C, Posch W. Comparative analyses of IgG/IgA neutralizing effects induced by threee COVID-19 vaccines against variants of concern. J Allergy Clin Immunol. 2022 Apr;149(4):1242-1252. PMID: 35093484
Selection of Funding
- CD-Labor für Invasive Pilzinfektionen; 2015-2022
- FWF, Doctoral programme of excellence, “HOROS”, ZFW012530, Host response in opportunistic infections, 2014-2023
- EU, Horizon2020, Marie Sklodowska Curie Action European Joint Doctorate “CORVOS”, 860044, Complement regulation & variations in opportunistic infections. 2019-2024
- FWF-Einzelprojekt P32329 Titel: Primäre Azoleresistenz in Mucormyzeten; 2019-2024
- MUI-Prototypenförderung 2022 Titel: SolNail; 2023
- FWF P33510: HIV-C entflieht Restriktion, nicht Sensing in DCs; 2020-2024
- FWF P34070: Treating SARS-CoV-2 infection in human 3D respiratory models; 2020-2024
- FWF P32329-B: Intrinsic azole resistance in mucormycetes; 2019-2023
- Fungal Dysbiosis in Pancreatic Ductal Adenocarcinoma Progression – The crosstalk with tumor microenvironment and its clinical implications. In Memoriam Dr. Gabriel Salzner Privatstiftung; 2021-2023
- FWF-Einzelprojekt P36922: Malassezia-induced immune-environment of pancreatic ductal adenocarcinoma; 2023-2026
Collaborations
Peter Garred, HOROS Guest Professor, Rikshospitalet, University of Copenhagen, Denmark
Maurizio Brigotti, University of Bologna, Italy
Brian Monk, Department of Oral Sciences, Division of Health Sciences, University of Otago, Dunedin, New Zealand
Oliver T. Keppler, Ludwig-Maximilians-Universität München, Munich, Germany
Teunis BH Geijtenbeek, Amsterdam UMC, Amsterdam Institute for Infection & Immunity, Amsterdam, The Netherlands
Oliver Cornely, Klinik I für Innere Medizin, Uniklinik Köln, Köln, Germany