Innrain 80/82
6020 Innsbruck
Fax: +43 512-9003-73110
Email: klaus.scheffzek@i-med.ac.at
Website: https://www.i-med.ac.at/imcbc/molecularcellbiologyfolder/molcellbiol.html
Research Branch (ÖSTAT Classification)
106041, 301904, 301303, 301902, 302040
Keywords
biochemistry, bioethics, biomolecular crystallography, ether lipid metabolism, high pressure liquid chromatography, immuno-biology, plasmalogens, protein purification, signal transduction/regulation, and Structural biology
Research Focus
We study the structure and function of disease-associated proteins and are currently focusing on Neurofibromatosis type I, chronic heart failure and Covid-19. Another focus is ether lipid metabolism, the biochemistry and physiological roles of its enzymes and metabolites. We also aim at understanding the biochemistry and clinical immunology of pteridine and tryptophan metabolism. In addition, we study bioethical, cultural and social issues of novel technologies in biomedicine.
General Facts
The unit consists of six principal investigators who study the impact of biomolecular systems on human health and disease. Our research areas include pteridine and lipid metabolism as well as intracellular signal transduction and its regulation, particularly in the context of G-proteins as well as drug targeting of viral infections.
The spectrum of our implemented methods includes biochemical techniques such as FPLC/HPLC for preparative protein purification and biochemical analysis, eukaryotic cell cultures and model organisms, various biophysical as well as bioanalytical methods and biomolecular x-ray crystallography.
We consider teaching to be a major responsibility in the education of young scientists and medical students and organise principal courses in several MUI curricula.
In addition, we investigate bioethical, social and cultural dimensions of novel biotechnologies in biomedicine, chair the inter-institutional and interdisciplinary bioethics network ethucation (http://www.i-med.ac.at/ethucation/index.html.en) established in 2007 and recently participated in implementing compulsory ethics teaching in all three sections of the medical curriculum (see: https://www.i-med.ac.at/medizinethik-lehre/).
Research
Structural Biology: Structure and Interactions of the Neurofibromatosis Type1 Protein, Mechanisms of G-Protein Complexes
Klaus Scheffzek & Theresia Dunzendorfer-Matt
We aim to understand the mechanisms of neurofibromatosis type 1 (NF-1), a genetic disease with a relatively high incidence. NF-1 patients have an increased risk of developing tumours and present with a variety of developmental defects. Mutations in the NF1 gene cause loss of function of the encoded protein neurofibromin, a giant RasGAP. Our long-term goal is to define the functional spectrum of neurofibromin in as much detail as possible. In continuation of previous work defining neurofibromin interacting proteins (Dunzendorfer-Matt et al., PNAS 2016), we study the structure of neurofibromin by cryo-electron microscopy (EM) and describe active and inactive forms potentially regulated by small molecules (Chaker-Margot et al., Mol Cell. 2021).
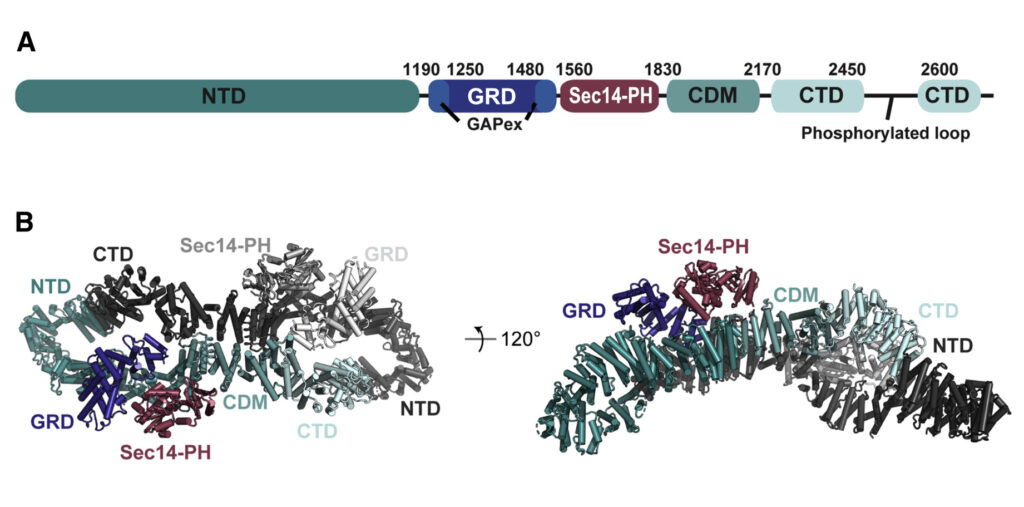
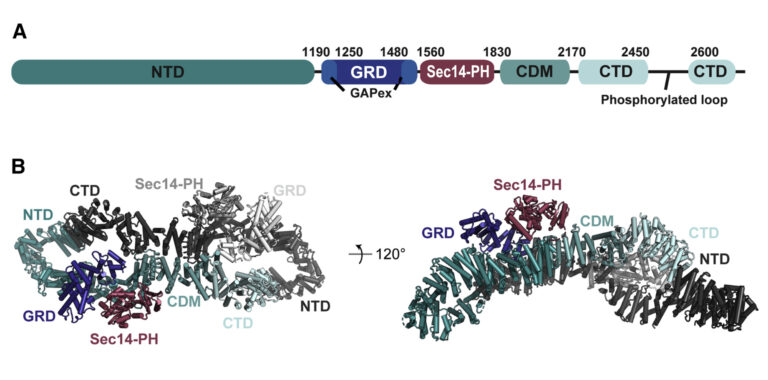
(A) Domain organisation of neurofibromin: NTD (N-terminal domain, dark teal), GRD (GAP-related domain, dark blue), GAPex (GAP-extra domain, blue), Sec14-PH (secretory protein 14-pleckstrin homology-like module, raspberry), CDM (central dimerization module, teal), CTD (C-terminal domain, light blue), interrupted by a loop with reported phosphorylation sites. (B) Cryo-EM based structural model of neurofibromin. The domains of one protomer are coloured as in (A), the second protomer is shown in shades of grey (figure by Chaker-Margot, see Chaker-Margot et al. Mol Cell. 2022 for scientific background).
Additional research involves a structure-based drug screening approach, where we aim at the determination of the high resolution crystal structure of the main SARS-CoV2 protease in complex with potential drug candidates. Compounds will also be screened for their inhibitory potential in biophysical, biochemical and cellular assays (International collaboration with Manfreid Weiss / DE and Vladimír Kryštof / CZ funded by FWF project I 5406).
Neuropsychoimmunology
Dietmar Fuchs
Mood changes and depression are common in patients suffering from inflammatory disorders, such as viral infections, autoimmune syndromes, malignant tumour diseases and adiposity. Although the pathogenesis of typical symptoms remains unclear, several of our studies have shown a correlation between neuropsychiatric deviations and elevated concentrations of neopterin and increased tryptophan breakdown (Kyn/Trp) in blood samples. These findings appear to have special relevance in neuropsychoimmunology and for the outcome of COVID-19. Elevated blood phenylalanine levels and higher phenylalanine to tyrosine ratios were observed in these patients. The combination of the Kyn/Trp ratio with the Phe/Tyr ratio can be especially helpful when deciding which treatments are more likely to be useful in the individual patient. The effect of nutrition and lifestyle (e.g. physical exercise and sport) on these immuno-biochemical pathways were presented as a focal point of our research. In patients suffering from otherwise treatment-resistant depression, repetitive transcranial magnetic stimulation (rTMS) has some beneficial effect and this is associated with a positive influence on phenylalanine metabolism, as shown in our collaboration with Kepler University (Linz).
Several clinical and in vitro studies using the model of freshly isolated peripheral blood mononuclear cells were performed and more than 60 papers were published between 2020 and 2022 in a worldwide collaboration with different research groups.
Ether Lipid Metabolism: Novel Gene Assignments and Research Tools
Katrin Watschinger, Gabriele Werner-Felmayer, Georg Golderer and Ernst R. Werner
Building on 40 years of pteridine research at the institute, we have been focusing on ether lipid metabolism for more than 15 years. The degradation of ether lipids requires alkylglycerol monooxygenase (TMEM195, AGMO), an enzyme that is dependent on the cofactor tetrahydrobiopterin.
In 2010, we assigned a sequence to AGMO. For the first time, we established a functional connection between tetrahydrobiopterin and lipid metabolism in intact cells.
When it loses its function, fatty aldehyde dehydrogenase (FALDH) – the subsequent enzyme in ether lipid metabolism – causes a severe, rare, hereditary disease: Sjögren-Larrson Syndrome. We used biochemical and structural methods to characterise the human fatty aldehyde dehydrogenase and found that a special structural feature of this enzyme, a gatekeeper helix, is responsible for its specificity to fatty aldehydes.
We have also recently succeeded in the genetic characterisation of plasmanylethanolamine desaturase (TMEM189, PEDS1), the enzyme that introduces the crucial double bond into plasmalogens, major glycerophospholipids in our brains and immune cell membranes. We have developed novel assays to characterise this reaction. Currently, we are investigating the physiological roles as well as the biochemical properties of AGMO and PEDS1 in various in vitro and in vivo models.
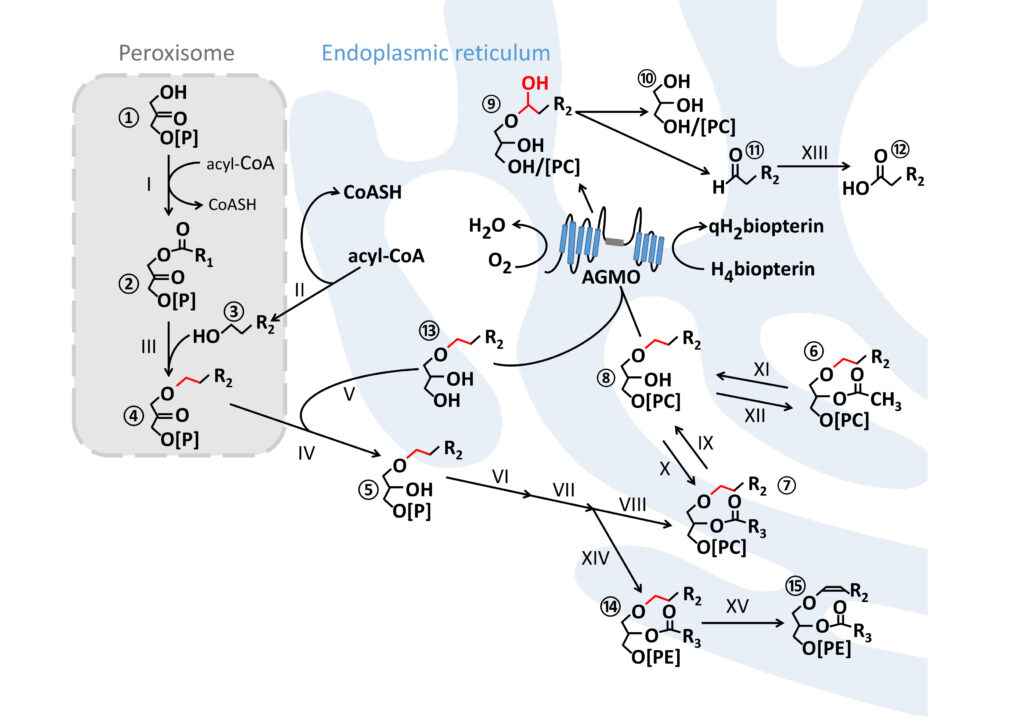
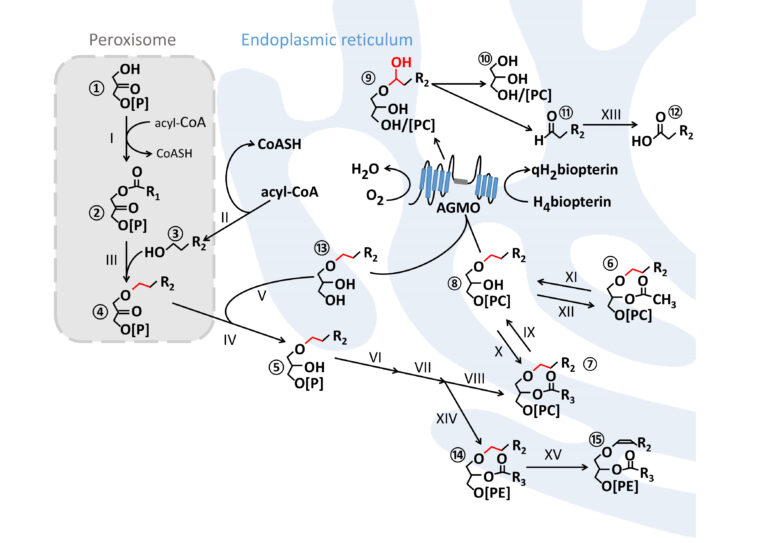
Ether lipid metabolism
Ether lipid synthesis is initiated in peroxisomes and further maturation steps take place at the endoplasmic reticulum. AGMO cleaves the ether bond (red) at the sn-1 of free alkylglycerols ⑬ or lyso-alkylglycerophospholipids ⑧ by using tetrahydrobiopterin (H4biopterin) as essential co-factor. This reaction creates a hemiacetal ⑨ which rearranges to the glycerol derivative ⑩ and a toxic fatty aldehyde ⑪ which is converted to the corresponding fatty acid ⑫ by fatty aldehyde dehydrogenase (XIII, FALDH, EC 1.2.1.48). Plasmanylethanolamine ⑭ is the substrate for PEDS1 (XV, EC 1.14.99.19), the enzyme that introduces the characteristic vinyl ether bond of plasmalogens ⑮. R = carbon side chain; R1 = mostly 15 or 17 atoms (attached with an acyl-bond); R2 comprises 14 or 16 atoms (attached with an alkyl-bond); R3 contains at least 15 atoms with one or several double bonds (attached with an acyl-bond).
Bioethics
Gabriele Werner-Felmayer
Focussing on bioethical, cultural and social dimensions in biomedicine, main topics of research include new technologies in the context of assisted reproductive technologies and prevailing deterministic views in genetics/genomics and medicine. In the context of organ transplantation, we study consent models and body concepts. Another focus is on ethical issues of data-driven medicine and the use of artificial intelligence. Activities include membership of Austria’s bioethics commission since 2017, chairing the inter-institutional and interdisciplinary bioethics network ethucation (http://www.i-med.ac.at/ethucation/index.html.en) established in 2007, implementing compulsory ethics teaching in the medical curriculum (with ao. Univ. Prof. Dr. Barbara Friesenecker, MUI, and Univ. Prof. Dr. Georg Gasser, University of Augsburg, Germany, see: https://www.i-med.ac.at/medizinethik-lehre/) and contributing to the Eurolife dataethics project (https://www.dataethics-eurolife.eu/).
Pictures
Selected Publications
- Structural basis of activation of the tumor suppressor protein neurofibromin. Chaker-Margot, M., Werten, S., Dunzendorfer-Matt, T., Lechner, S., Ruepp, A., Scheffzek, K., Maier, T. Mol Cell. 82, 2022, 1288-1296.
- Essential role of a conserved aspartate for the enzymatic activity of plasmanylethanolamine desaturas, Ernst R Werner, Monica L Fernández-Quintero, Nicolas Hulo, Georg Golderer, Sabrina Sailer, Katharina Lackner, Gabriele Werner-Felmayer, Klaus R Liedl, Katrin Watschinger, Cell Mol Life Sci. 2022 Mar 28;79(4):214. doi: 10.1007/s00018-022-04238-w.
- When the genome bluffs: a tandem duplication event during generation of a novel Agmo knockout mouse model fools routine genotyping. Sailer S, Coassin S, Lackner K, Fischer C, McNeill E, Streiter G, Kremser C, Maglione M, Green CM, Moralli D, Moschen AR, Keller MA, Golderer G, Werner-Felmayer G, Tegeder I, Channon KM, Davies B, Werner ER, Watschinger K. Cell Biosci. 2021 Mar 16;11(1):54. doi: 10.1186/s13578-021-00566-9.
- Attitudes of European students towards family decision-making and the harmonisation of consent systems in deceased organ donation: a cross-national survey. Molina-Pérez A, Werner-Felmayer G, Van Assche K, Jensen AMB, Delgado J, Flatscher-Thöni M, Hannikainen IR, Rodriguez-Arias D, Schicktanz S, Wöhlke S. BMC Public Health. 2022 Nov 15; 22(1):2080. doi: 10.1186/s12889-022-14476-z.
- Leblhuber F, Geisler S, Ehrlich D, Steiner K, Kurz K, Fuchs D. High frequency repetitive transcranial magnetic stimulation improves cognitive performance parameters in patients with Alzheimer’s Disease. Curr Alzheimer Res 2022;19, 681-688.
Selection of Funding
- NECESSITY-Neue SARS-CoV-2 modulierende chemische Konstrukte, Austrian Science Fund (FWF), Klaus Scheffzek
- Dissecting the role of plasmalogens in ether lipid-associated pathologies, Austrian Science Fund (FWF), Katrin Watschinger
- Ether lipid metabolism & oxidative damage in Crohn`s disease, Austrian Science Fund (FWF), Katrin Watschinger
Collaborations
- Thomas Wieland, Medical Faculty Mannheim, Heidelberg University, Germany
- Manfred Weiss, Helmholtz-Zentrum Berlin, Germany
- Vladimír Kryštof, Palacký University Olomouc, Czech Republic
- Eduard Stefan, Leopold Franzens University, Innsbruck, Austria
- Thomas Rausch, University Heidelberg, Heidelberg Germany
- Keith Channon, Jonathan Hodgkin, University of Oxford, United Kingdom
- Johannes Berger, Medical University Vienna, Vienna, Austria
- Markus A. Keller, Medical University of Innsbruck, Innsbruck, Austria
- Stefan Coassin, Medical University of Innsbruck, Innsbruck, Austria
- Manuel Maglione, Medical University of Innsbruck, Innsbruck, Austria
- Wendy Chung, Columbia University, New York, USA
- Frederic M. Vaz, University of Amsterdam, Amsterdam, The Netherlands
- Lucile Capuron, University of Bordeaux, France
- Magnus Gisslen, Lars Hagberg, Östra University Hospital, Gothenburg, Sweden
- Harald Mangge, Eva Reininghaus, Medizinische Universität Graz, Austria
- Teo T Postolache, University of Baltimore, MD, USA
- Richard W Price, Institut of Neurology, San Francisco General Hospital, UCSF, USA
- Barbara Prainsack, University of Vienna, Austria and Kings College London, UK
- Silke Schicktanz University Medicine Goettingen; Germany
- International Chair in Bioethics, University of Porto, Porto, Portugal
- Research Center Medical Humanities, Leopold Franzens University, Innsbruck, Austria