Anichstraße 35
Haus 2
6020 Innsbruck
Fax: +43 50 504 23538
Email: herbert.tilg@i-med.ac.at
Website: http://www.inneremed1.tirol-kliniken.at/
Research year
Research Branch (ÖSTAT Classification)
3518, 3510, 3516, 3536, 3509
Keywords
endocrinology, Gastroenterology, hepatology, Inflammation, inflammatory bowel disease, metabolism, microbiota, non-alcoholic fatty liver disease, and nutrition
Research Focus
The department focuses on translational research in the fields of gastroenterology, hepatology, endocrinology and metabolism with a special focus on inflammatory mechanisms. The overall objective of our scientific activities is to gain improved insights into the pathophysiology of prevalent diseases, such as inflammatory bowel disease, metabolic and genetic liver diseases, obesity, type 2 diabetes and atherosclerosis. Better knowledge will help to improve clinical management of these patients.
General Facts
The Department of Internal Medicine I focuses on the specific medical areas of gastroenterology, hepatology, endocrinology and metabolism. Our division has approximately 45 employees and many members, including students, are involved both in clinical work and in research. Our laboratories are excellently equipped and our researchers are able to conduct state-of-the-art research in the field of cellular and molecular work. The common aims of our research activities are to improve knowledge in the respective disease areas and to improve patient care.
In our department, we are host to two Christian Doppler research laboratories and one funded by the ERC starting grant:
- ERC STG laboratory on mucosal immunology (Univ.-Prof.Dr. Timon E. Adolph)
- Christian Doppler research laboratory on insulin resistance (Univ.-Prof.in Dr.in Kaser) (completed)
- Christian Doppler research laboratory on iron and phosphate biology (Univ.-Prof. Dr. Zoller)
For a number of years, our research has been funded by the Austrian Research Promotion Agency (FFG), Austrian Science Fund (FWF), Christian Doppler Research Association (CDG) and European Union (FP7). We have published our research in highly respected international journals, such as Nature Reviews Immunology, Nature Communications, New England Journal of Medicine, Cell Host Microbe, Gastroenterology, Gut, JAMA and many others.
Research
Microbiota and Gastrointestinal Disorders
Herbert Tilg
This research group has been involved in microbiome research for over a decade. Several projects have been completed, for example demonstrating a key role of Akkermansia muciniphila in alcoholic liver disease (Grander C. Gut 2018; 67: 891). A major microbiome project has been published recently (Si Y et al. Nat Aging 2022). In it, we showed that long-term life history affects the gut microbiota, thereby providing insights into how lifestyle variables influence and maintain a healthy gut microbiota in later life.
The following microbiome projects are currently in progress:
- The role of gut and liver microbiome in hepatocellular carcinoma (in collaboration with K. Aden, Kiel, Germany)
- CHRIS study: Type 2 diabetes and NAFLD: pathophysiological relevance of gut microbiome and metabolites (study conducted in collaboration with EURAC and V. Temaroli, Gothenburg, Sweden)
In recent years, several highly ranked papers have been published and Dr. Maria Effenberger has received the most prestigious award from the Austrian Society for Gastroenterology and Hepatology (Wewalka Award) in 2022. Herbert Tilg has been a much cited researcher (HCR) 2020-2022 (H-Index: 95).
Intestinal Inflammation
Timon Adolph
At the Department of Internal Medicine I Gastroenterology laboratory, funded by the ERC, we aim to resolve the impact of diet on inflammatory diseases of the gastrointestinal tract and particularly in understanding the pathophysiology of inflammatory bowel disease. In a stand-alone project funded by the FWF in 2020, we identified the role of intestinal epithelial glutathione peroxidase 4 (GPX4) in intestinal homeostasis. We discovered that a 3 months long Western diet induces a phenotype similar to Crohn’s disease in GPX4-deficient mice. In our work, published in Nature Communications (Mayr L. et al., Nature Communications 2020, 11, 1775), we report that GPX4 restricts a cytokine response – specifically interleukin 6 (IL-6) and C-X-C Motif Chemokine Ligand 1 (CXCL1) expression – from intestinal epithelial cells (IECs), which is elicited by dietary polyunsaturated fatty acids (PUFAs). As such, we have identified that dietary PUFAs (contained in meat, eggs and oils) can trigger a phenotype similar to Crohn’s disease in mice with reduced epithelial GPX4 activity. We further identified a mechanism of how this dietary polyunsaturated fatty acid-induced phenotype is triggered in the intestinal epithelium, which we published in Gastroenterology 2022 (Schwärzler J and Mayr L et al Gastroenterology 162(6):1690-1704). Moreover, we recently proposed the concept of diet-induced metabolic gut inflammation in Nat Rev Gastroenterol & Hepatol. (Adolph TE et al Nat Rev Gastroenterol Hepatol. 2022 19(12):753-767).
Current studies aim at delineating mechanistic aspects of autophagy (covered by an FWF stand-alone project until 2023) and other cellular hubs (funded by the ERC until 2027 and an FWF “Forschungsgruppen (FG)” Grant) that may control dietary lipid-induced gut inflammation and its relevance in IBD. The funding allows the employment of 1 post-doc, 3 PhD students and several MD students (marginal employment) over the next years. Scientific excellence was also highlighted by the election of Timon Adolph to a member of the Österreichische Akademie der Wissenschaften (Young ÖAW, 2022) and a rising star award of the European Society of Gastroenterology (2023). Our science is also brought to public attention in Austrian and German news magazines (“Die Presse”, “ORF”, “Der Standard”, “Tiroler Tageszeitung”, “Österreichische Ärztezeitung”, “Der Spiegel”, “Radio Berlin Brandenburg” and the TV service “Bayrischer Rundfunk”).
Based on this success, young researchers in the Gastroenterology laboratory were awarded with visible research prizes and were able to secure further funding, for example Dr. Felix Grabherr received a Wissenschaftspreis des Landes Vorarlberg and a project funded by the European Crohn’s and Colitis Organisation, and Dr. Julian Schwärzler received a Liechtensteinpreis (MUI) a Zukunftspreis from the German Society of IBD and a “Wissenschaftspreis” by the Austrian Society of Gastroenterology & Hepatology.
Hepatology
Heinz Zoller
One main research area is the genetic basis of liver diseases, with a particular interest in genetic factors that predispose individuals to the development of iron overload and subsequently end-stage liver disease. Recently, this knowledge has also been applied in research on COVID19. In collaboration with the international COVID-19 Host Genetics Initiative, they have identified several genetic markers associated with a severe course of COVID19 (COVID-19 Host Genetics Initiative. Nature. 2021 Dec;600(7889):472-477 and Degenhardt F et al Hum Mol Genet. 2022 Nov 28;31(23):3945-3966).
Other research areas of the group include liver transplantation, viral hepatitis delta and liver cirrhosis, where clinical and translational studies aim to evaluate better prognostic models and new treatments for these conditions (Jachs M. Aliment Pharmacol Ther. 2022 Jul;56(1):144-154).
The hepatology group has also made important contributions to the study of iron metabolism and iron-related disorders by analysing the role of genetic factors in iron overload disorders, such as hereditary hemochromatosis, which is a common genetic disorder that causes the accumulation of excess iron in the body. Based on this long-standing research, Heinz Zoller and Benedikt Schäfer have led a European consortium that devolved the European Association for the Study of the Liver’s clinical practice guidelines on hemochromatosis (EASL J Hepatol. 2022 Aug;77(2):479-502)
As certain liver diseases can also affect the lungs and the brain, the team at the Hepatology laboratory is also interested in the role of liver-secreted proteins in the function of extrahepatic organs. This applies to the study of the genetic condition alpha-1-Antitrypsin deficiency with respect to the lung and aceruloplasminemia with respect to the brain. Ceruloplasmin protects the brain against oxidative stress and inflammation. Current research aims at developing rodent disease models for brain iron accumulation that is typical for aceruloplasminemia as the basis for gene therapies of this vexing condition. To achieve this aim, automated volume segmentation of the brain has been applied in another liver disease, where marked but subclinical neurodegeneration has been found in patients with Wilson disease (Viveiros A. Hepatology. 2021 Aug;74(2):1117-1120)
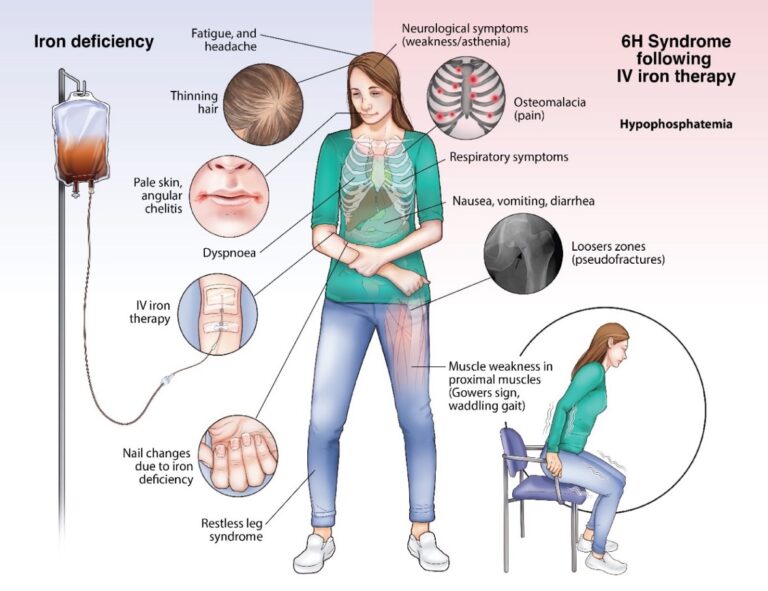
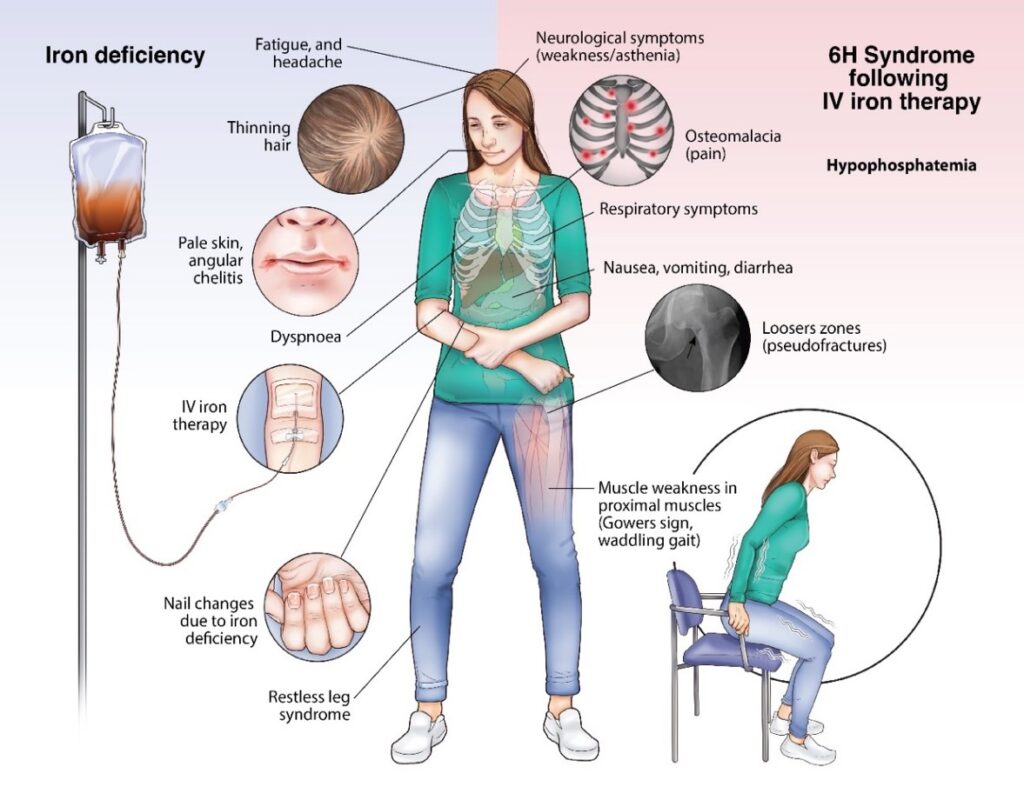
Based on its long-standing interest in iron metabolism, the Hepatology Research Group has also conducted extensive research into the mechanisms underlying iron-induced hypophosphatemia, with the goal of developing new strategies for the prevention and treatment of this condition. Their research has focused on the role of fibroblast growth factor 23 (FGF23), a hormone that regulates phosphate metabolism in the body (Zoller H Gut. 2023 Apr;72(4):644-653). The complex changes elicited by the intravenous iron formulation carboxymaltose, the Hepatology Research Group recently coined the term “6H syndrome”
Endocrinology and Metabolism
Susanne Kaser
Diabetes mellitus is one of the top ten causes of death worldwide, affecting more than 800,000 people in Austria. Another 300,000 people in Austria are thought to suffer from prediabetes and are therefore at very high risk of being diagnosed with diabetes within the next few years. Strong genetic predisposition, over-nutrition or unhealthy diet, a sedentary lifestyle and smoking are well-known risk factors for type 2 diabetes, which accounts for more than 90% of all diabetes cases.
Prevention and treatment of insulin resistance/type 2 diabetes and target organ damage:
We are especially interested in studying the effects of dieting, overeating, obesity and hyperglycaemia on the development of insulin resistance/type 2 diabetes and target organ damage. In a recent paper (Radlinger B et al, Diabetologia, 2023; 66:754), we were able to show preventive effects of empagliflozin treatment on the development of obesity, insulin resistance and fatty liver disease in a high risk setting for the first time. Quite remarkably, we also demonstrated diet-independent beneficial effects of empagliflozin treatment on mitochondrial function in skeletal muscle, suggesting that empagliflozin exerts protective effects on mitochondrogenesis regardless of overweight, insulin resistance and Western diet. These data provide new insights into pleiotropic effects of SGLT-2 inhibitors but, even more importantly, show protective effects in a primary prevention setting. In an earlier study, we demonstrated that empagliflozin exerts cardio-protective effects regardless of its capacity to reduce blood glucose and body weight (Radlinger B et al, Sci Rep, 2020) by improving cardiac insulin signalling, mitochondrial ultrastructure and mass as well as cardiac inflammation. These data also revealed novel insights into pathophysiology and treatment of diabetic cardiomyopathy.
In a recent work, we were able to identify apolipoprotein A5 as critical determinant of fructose-induced metabolic disease and hepatic steatosis (Ress C et al, Nutr Metab Cardiovasc Dis, 2021; 32:972) suggesting apolipoproteinA5 as a novel treatment target in diet-induced metabolic disease.
Based on previous studies by our group showing gender-specific effects of fructose-enriched diets, high-fat diets and Western diets on adipose tissue (Dobner J et al, J Nutr Biochem, 2017) we wanted to investigate whether switching the diets from a Western to a more beneficial diet without caloric restriction was sufficient to restore energy metabolism and tissue-specific changes. In fact, we found that switching the diet without caloric restriction can improve adipose tissue inflammation, but is unable to restore the thermogenic capacity of brown adipose tissue and the addition of white adipose tissue, suggesting that the Western diet at least partially aggravates adipocyte energy metabolism in the long-term (Folie S et al, J Nutr Biochem, 2022).
Atherosclerosis
Andreas Ritsch
We are interested in the role of reverse cholesterol transport in atherosclerosis. Recently, we investigated structure-function-disease relationships of HDL in healthy subjects and in patients with diabetes (T2DM) or coronary heart disease (CHD). We showed that the different cellular functions of HDL correlate only weakly with each other and are determined by different structural components (Cardner M, et al., JCI Insight. 2020 Jan 16; 5(1): e131491). In pharmacological studies of ApoE knockout rabbits, an animal model for atherosclerosis, we showed that Matcha green tea enhances atherosclerosis in these animals, due to impaired reverse cholesterol transport (Monika Hunjadi, et al., manuscript submitted). In a large clinical study (Young Finns study), we showed an inverse correlation between HDL cholesterol efflux capacity and subclinical cardiovascular risk markers in young adults (Hunjadi M, et al., Scientific Reports. 2020 Nov 5; 10(1): 19223.). Studies of 2468 participants of the LURIC study have shown that cholesterol efflux is associated with HDL composition as well as an inflammatory burden in patients referred for coronary angiography and that this is an inverse predictor of cardiovascular mortality, independently of HDL cholesterol (Ritsch A, et al., Biomedicines. 2020 Nov 21; 8(11): 524).
Pictures
Selected Publications
Research team Tilg:
- Tilg H, Adolph TE, Trauner M. Gut-liver axis: Pathophysiological concepts and clinical implications. Cell Metab. 2022 Nov 1;34(11):1700-1718. doi: 10.1016/j.cmet.2022.09.017. Epub 2022 Oct 7.
- Effenberger M et al, IL-11 drives human and mouse alcohol-related liver disease. Gut. 2023 Jan; 72:168-179. doi: 10.1136/gutjnl-2021-326076. Epub 2022 Apr 03.
- Si J,… Kiechl S, Tilg H et al . Long-term life history predicts current gut microbiome in a population-based cohort study. Nature aging. 2022 Oct 14;doi: 10.1038/s43587-022-00286-w.
Research team Adolph:
- Adolph TE, Meyer M, Schwärzler J, Mayr L, Grabherr F, Tilg H. The metabolic nature of inflammatory bowel diseases. Nat Rev Gastroenterol Hepatol. 2022 Dec;19(12):753-767. doi: 10.1038/s41575-022-00658-y. Epub 2022 Jul 29.
- Zollner A, Koch R, Jukic A, Pfister A, Meyer M, Rössler A, Kimpel J, Adolph TE, Tilg H. Postacute COVID-19 is Characterized by Gut Viral Antigen Persistence in Inflammatory Bowel Diseases. Gastroenterology. 2022 Aug;163(2):495-506.e8. doi: 10.1053/j.gastro.2022.04.037. Epub 2022 May 1.
- Schwärzler J, Mayr L, Vich Vila A, Grabherr F, Niederreiter L, Philipp M, Grander C, Meyer M, Jukic A, Tröger S, Enrich B, Przysiecki N, Tschurtschenthaler M, Sommer F, Kronberger I, Koch J, Hilbe R, Hess MW, Oberhuber G, Sprung S, Ran Q, Koch R, Effenberger M, Kaneider NC, Wieser V, Keller MA, Weersma RK, Aden K, Rosenstiel P, Blumberg RS, Kaser A, Tilg H, Adolph TE. PUFA-Induced Metabolic Enteritis as a Fuel for Crohn’s Disease. Gastroenterology. 2022 May;162(6):1690-1704. doi: 10.1053/j.gastro.2022.01.004. Epub 2022 Jan 12.
Research team Zoller:
- Covid-19 Host Genetics Initiative. Nature. 2021 Dec; 600(7889):472-477.
- Degenhardt, F et al. Detailed stratified GWAS analysis for severe COVID-19 in four European populations. Hum Mol Genet. 2022 Nov 28; 31(23):3945-3966.
- Zoller H, Schaefer B et al. EASL Clinical Practice Guidelines on haemochromatosis. J Hepatol. 2022 Aug; 77(2)479-502.
- Viveiros A. Neurodegeneration in Hepatic and Neurologic Wilson’s Disease. Hepatology. 2021 Aug; 74(2):1117-1120.
- Zoller H. Hypophosphataemia following ferric derisomaltose and ferric carboxymaltose in patients with iron deficiency anaemia due to inflammatory bowel disease (PHOSPHARE-IBD): a randomised clinical trial. Gut. 2023 Apr; 72 (4):644-653.
- Schaefer, B. Bone. Hypophosphatemia after intravenous iron therapy: Comprehensive review of clinical findings and recommendations for management. 2022 Jan; 154-116202.
Research team Kaser:
- Radlinger B et al & Kaser S. Empagliflozin protects mice against diet-induced obesity, insulin resistance and hepatic steatosis. Diabetologia, 2023
- Folie S et al & Kaser S. Changing the dietary composition improves inflammation but not adipocyte thermogenesis in diet-induced obese mice. J Nutr Biochem, 2022
- Sourij H et al & Kaser S. COVID-19 fatality prediction in people with diabetes and prediabetes using a simple score upon hospital admission. Diabetes Obes Metab 2020
- Radlinger B, Ress C, Kaser S, Tilg H et al. Empagliflozin protects mice against diet-induced obesity, insulin resistance and hepatic steatosis. Diabetologica. 2023; 66:754-767. Epub: 2022 Dec 17
- Ress C et al & Kaser S. Apolipoprotein A5 controls fructose-induced metabolic dysregulation in mice. Nutr Metab Cardiovasc Dis 2022
Research team Ritsch:
- Cardner M, Yalcinkaya M, Goetze S, Luca E, Balaz M, Hunjadi M, Hartung J, Shemet A, Kränkel N, Radosavljevic S, Keel M, Othman A, Karsai G, Hornemann T, Claassen M, Liebisch G, Carreira E, Ritsch A, Landmesser U, Krützfeldt J, Wolfrum C, Wollscheid B, Beerenwinkel N, Rohrer L, von Eckardstein A. Structure-function relationships of HDL in diabetes and coronary heart disease. JCI Insight. 2020 Jan 16;5(1):e131491.
- Hunjadi M, Lamina C, Kahler P, Bernscherer T, Viikari J, Lehtimäki T, Kähönen M, Hurme M, Juonala M, Taittonen L, Laitinen T, Jokinen E, Tossavainen P, Hutri-Kähönen N, Raitakari O, Ritsch A. HDL cholesterol efflux capacity is inversely associated with subclinical cardiovascular risk markers in young adults: The cardiovascular risk in Young Finns study. Sci Rep. 2020 Nov 5;10(1):19223. doi: 10.1038/s41598-020-76146-7.
Selection of Funding
- FWF No.: FT33070 (Timon Adolph)
- Christian-Doppler-Research Laboratory for insulin resistance (Univ.-Prof.in Dr.in Kaser) (completed)
- Christian-Doppler-Research Laboratory for Iron and Phosphate Biology (Univ.-Prof. Dr. Zoller)
- Funding is also supported by the excellence initiative VASCage (Centre for Promoting Vascular Health in the Ageing Community), an R&D K-Centre (COMET program – Competence Centers for Excellent Technologies) funded by the Austrian Ministry for Transport, Innovation and Technology, the Austrian Ministry for Digital and Economic Affairs and the federal states Tyrol, Salzburg and Vienna. (Herbert Tilg)
Collaborations
- Arthur Kaser, Cambridge
- PD Dr. Konrad Aden, Kiel
- Richard Steven Blumberg, Boston Harvard Medical School, Gastroenterology, Hepatology & Endoscopy
- Michael Roden, Düsseldorf, Endocrinology
- Erwin Wagner, PhD, Medical University Vienna
- Michael Trauner, Medical University Vienna
- Percy A. Knolle, TUM Technical University Munich