Anichstraße 35
Frauenkopfklinik Haus Nr 3
6020 Innsbruck
Fax: +43 512 504-23055
Email: Christian.Marth@i-med.ac.at
Website: https://www.i-med.ac.at/patienten/ukl_gynaekologie_geburtshilfe.html https://frauenheilkunde-innsbruck.tirol-kliniken.at
Research Branch (ÖSTAT Classification)
302022, 302017, 30205, 301306
Keywords
Biobanking, Biomarker-identification, Clinical studies, Drug testing, Gynaecologic Oncology, Senology, and Translational Research
Research Focus
Clinical and preclinical studies in cancers specific to women, with regard to prevention, diagnosis, treatment and follow-up care
Translational research:
Biomarker identification in breast and gynaecological malignancies
Specialist interest in increasing understanding of the development of female cancers
Drug testing
Collection of bio-samples (tissues, serum, plasma, ascites…)
Pregnancy research
General Facts
Most of the clinical studies conducted by the Dept. are in collaboration with other task forces and research groups, both within the clinic and internationally. Also located at the Dept. is the AGO Studienzentrale as the trial office of the non-profit organisation AGO Austria (Austrian gynaecological oncology working group). The aim of our Dept. is to promote and advance gynaecological (especially ovarian and breast) cancer research with regard to prevention, diagnosis, treatment and follow-up care. Current trials involve specific therapies such as poly(adenosine diphosphate-ribose) polymerase (PARP) or PI3K inhibitors, immunotherapy with checkpoint inhibitors, as well as novel cytotoxic substances and antibody-drug conjugates (ADCs) and surgical trials.
Preclinical studies are mainly performed in the Laboratory for Clinical Biochemistry (Head: Heidi Fiegl). The laboratory is ISO 9001:2015 certified and houses the Dept.’s biobank, which consists of fresh frozen (FF) tissue samples from over 1,000 patients, ascites samples from over 1,500 patients (FF tissue biobank) and serum or plasma samples (pre-therapeutic samples and samples from the follow-up period) from over 3,000 patients (serum biobank). Biobanking has been operated here since the 1980s.
Research
Clinical Trials: Gynaecological Oncology
Leader: Christian Marth
A number of gynaecological cancer trials (surgical and therapeutic trials) focusing on assessing the efficacy and safety of different types of treatment for ovarian (OC), endometrial (EC) and cervical cancer (CC) have been conducted. These treatments include targeted therapies such as tumour treatment fields using insulated transducer arrays, aromatase inhibitors, PI3K inhibitors, ADCs, angiogenesis inhibitors, poly(ADP-ribose) polymerase inhibitors and immunotherapy, especially checkpoint inhibitors for PD-1 and PD-L1.
Selected trials are described below:
- INNOVATE-3: A pivotal, randomised, controlled, open-label trial, designed to test the efficacy and safety of tumour treating fields generated by a medical device, the NovoTTF-100L(O) system, in combination with paclitaxel for patients with platinum-resistant OC.
- DUO-O: A phase III randomised, double-blind, placebo-controlled, multicentre study of durvalumab in combination with chemotherapy and bevacizumab, followed by maintenance durvalumab, bevacizumab and olaparib in newly diagnosed advanced OC patients.
- ATALANTE: A randomised, double blind, phase III study of atezolizumab versus placebo in patients with late relapse of OC, fallopian tube or peritoneal cancer treated with platinum-based chemotherapy and bevacizumab.
- MATAO: Maintenance therapy with aromatase inhibitor in epithelial OC: a randomised double-blind placebo-controlled multicentre phase III trial.
- EPIK-O: A phase III, multi-centre, randomised (1:1), open-label, active-controlled study to assess the efficacy and safety of alpelisib (BYL719) in combination with olaparib as compared to single agent cytotoxic chemotherapy, in participants with no germline BRCA mutation detected, platinum-resistant or refractory, high-grade (HG) serous OC (HGSOC).
- Ovar 2.29: Atezolizumab in combination with bevacizumab and chemotherapy versus bevacizumab and chemotherapy in recurrent OC – a randomised phase III trial.
- LEAP 001: A phase-III trial comparing an immunotherapy (pembrolizumab) and a targeted therapy (levatinib) with chemotherapy in patients with stage-III or IV EC or in patients whose cancer has returned following previous treatment.
- AtTEnd: Phase III double-blind randomised placebo controlled trial of atezolizumab in combination with paclitaxel and carboplatin in women with advanced/recurrent EC.
- ENGOT-en11: A phase 3, randomised, double-blind study of pembrolizumab versus placebo in combination with adjuvant chemotherapy with or without radiotherapy for the treatment of newly diagnosed high-risk EC after surgery with curative intent.
- ENGOT-cx11: A randomised, phase 3, double-blind study of chemotherapy with or without pembrolizumab for the treatment of high-risk, locally advanced CC.
Leader: Alexandra Ciresa-König, Andreas Widschwendter:
- PITVIN: Primary Imiquimod Treatment versus Surgery for Vulvar Intraepithelial Neoplasia. Multicentre, randomised, phase 3, non-inferiority clinical trial performed by the AGO at six hospitals in Austria. The study revealed that Imiquimod is a safe, effective and well accepted alternative to surgery for women with vulvar high-grade squamous intraepithelial lesions and can be considered as first-line treatment
Leader: Nicole Concin
- UPLIFT: A phase 1b/2, first-in-human, dose escalation and expansion study of XMT-1536 in patients with solid tumours likely to express NaPi2b.
- GANNET53: This trial consists of a multicentre Phase I dose-escalation/de-escalation part and a European-wide, multicentre, open-label, randomised Phase II part and assesses the safety and efficacy of the Hsp90 (heat shock protein 90) inhibitor Ganetespib in combination with weekly Paclitaxel in platinum-resistant OC patients.
- EUDARIO: This is a multicentre, open-label, three-arm randomised phase II trial assessing the safety and efficacy of Ganetespib in combination with Carboplatin and Niraparib, respectively, in platinum-sensitive OC patients.
Epithelial ovarian cancer (EOC) is the most lethal gynaecological malignancy. HGSOC, HG endometrioid and undifferentiated carcinomas are characterised by almost ubiquitous TP53 mutations (> 96% of patients). The 7th framework programme project GANNET53, funded by the European Commission, addresses the pressing need for more effective, innovative treatment strategies to improve the dismal survival rate in this major subgroup of EOC patients. The two clinical trials GANNET53 and EUDARIO apply a drug strategy inhibiting the central chaperone Hsp90 in TP53 mutant (mutp53) EOC. In the GANNET53 trial, this innovative Hsp90 inhibition mechanism is used to target the central driver of aggressiveness and metastatic ability of these EOC cancers – namely stabilise mutp53 –for degradation. In the EUDARIO trial, the concept of Hsp90 inhibition is exploited to crucially inhibit DNA repair by the rapid decay of key components of the Fanconi anaemia pathway as well as cell cycle checkpoint mediators following DNA damage by platinum-based chemotherapy.
Clinical Trials: Breast Cancer
Leaders: Christian Marth, Daniel Egle
The breast cancer (BC) care unit successfully participated in several clinical trials in 2021 and 2022, especially in trials using new targeted therapies like PARP-Inhibitors (Olaparib in eTNBC), ADCs (i.e. Trastuzumab-Deruxtecan and Sacitucumab-Govitecan) and checkpoint inhibitors (Atezolizumab and Pembrolizumab).
Below is a short selection of the most important clinical trials:
- ABCSG 45/ PARP-Inhibitor/Olaparib: A prospective, open, randomised, phase 2 study of Carboplatin/ Olaparib in the pre-operative treatment of patients with triple-negative primary BC and a positive homologous recombination deficiency (HRD) status.
- CHECKMATE07/Checkpoint Inhibitor/Nivolumab: A randomised, multicentre, double-blind, placebo-controlled phase 3 study of Nivolumab versus placebo in combination with neoadjuvant chemotherapy and adjuvant endocrine therapy in patients with high-risk, estrogen receptor- positive (ER+), human epidermal growth factor receptor 2- negative (HER2-) primary BC.
- KEYNOTE-B49/Checkpoint Inhibitor/Pembrolizumab: A randomised, double-blind, placebo-controlled, phase 3 study of Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for the treatment of chemotherapy-candidate hormone receptor-positive (HR+), HER2- locally recurrent inoperable or metastatic BC.
- ABCSG56/SASCIA/Antibody Drug Conjugate/Sacituzumab Govitecan: Phase 3 post-neoadjuvant study evaluating Sacituzumab Govitecan, an antibody drug conjugate in primary HER2-negative BC patients with high relapse risk after standard neoadjuvant treatment.
- DESTINY-BREAST06/Checkpoint Inhibitor/Trastuzumab Deruxtecan: A Phase 3, randomised, multicentre, open-label study of Trastuzumab Deruxtecan (T-DXd) versus investigator’s choice chemotherapy in HER2-low, HR+ positive BC patients whose disease has progressed on endocrine therapy in the metastatic setting.
- HER2CLIMB-02/Tyrosinkinase-Inhibitor/Tucatinib: Randomised, double-blind, phase 3 study of Tucatinib or placebo in combination with Ado-Trastuzumab Emtansine (T-DM1) for subjects with non-resectable locally-advanced or metastatic HER2+ BC.
Leader Christine Brunner:
Hair-Safe Study: Scalp Cooling for Hair Saving in Women Undergoing Chemotherapy: A significant proportion of BC patients still receive chemotherapy as part of their treatment. Chemotherapy-induced alopecia (CIA) has a very significant, negative impact on the patients’ quality of life. Therefore, a prospective study was initiated to investigate the efficacy of scalp cooling (SC) in different chemotherapy regimens and the recovery of hair during the follow-up period. SC significantly reduced CIA in BC patients, especially those receiving taxane monotherapy, but had no significant effect on hair regrowth after chemotherapy. Based on this study, SC can be recommended to be integrated into clinical practice.
Clinical trials: Pregnancy
Leader: Johanna Tiechl:
Screening for Open Spina Bifida in a Routine Clinical Setting at the First-Trimester Scan. In this prospective multicentre study of 4,755 women undergoing first trimester ultrasound examination, brainstem (BS) diameter, brainstem-to-occipital-bone (BSOB) distance and the cisterna magna (CM) were measured. The BS/BSOB ratio showed very good sensitivity and specificity for detecting open spina bifida. In cases with near normal values for the BS/BSOB ratio, the CM width may be helpful for further evaluation.
Translational research
A selection of the most important projects is shown:
Leader: Nicole Concin
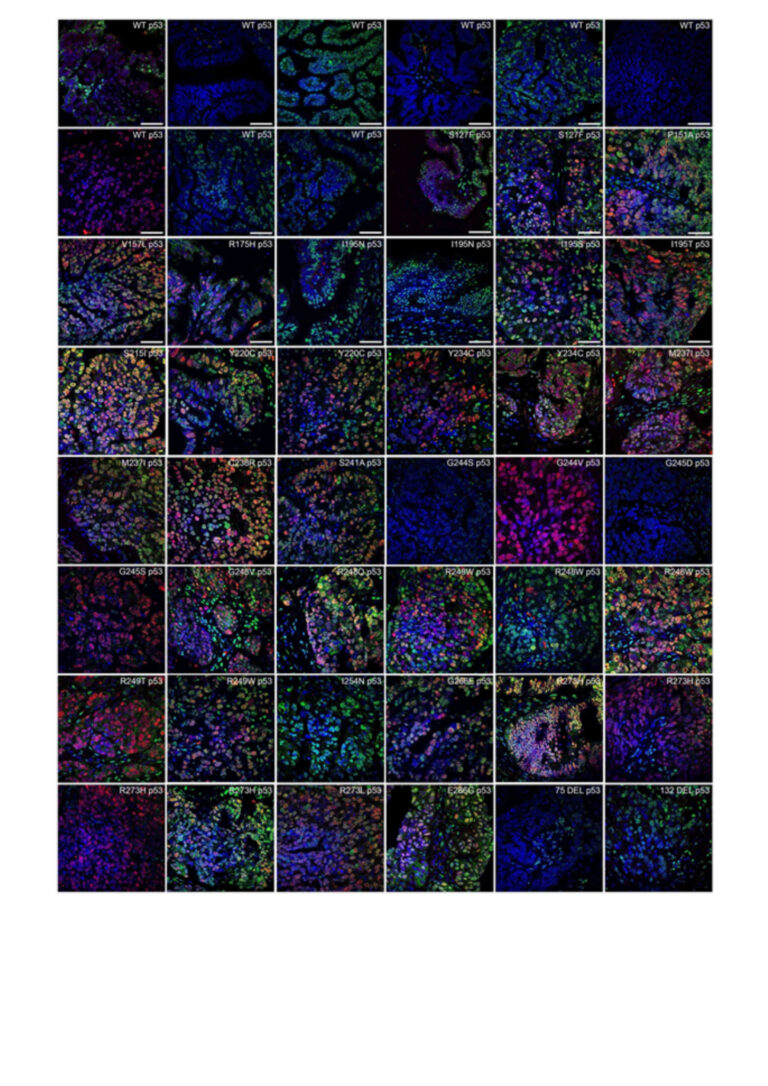
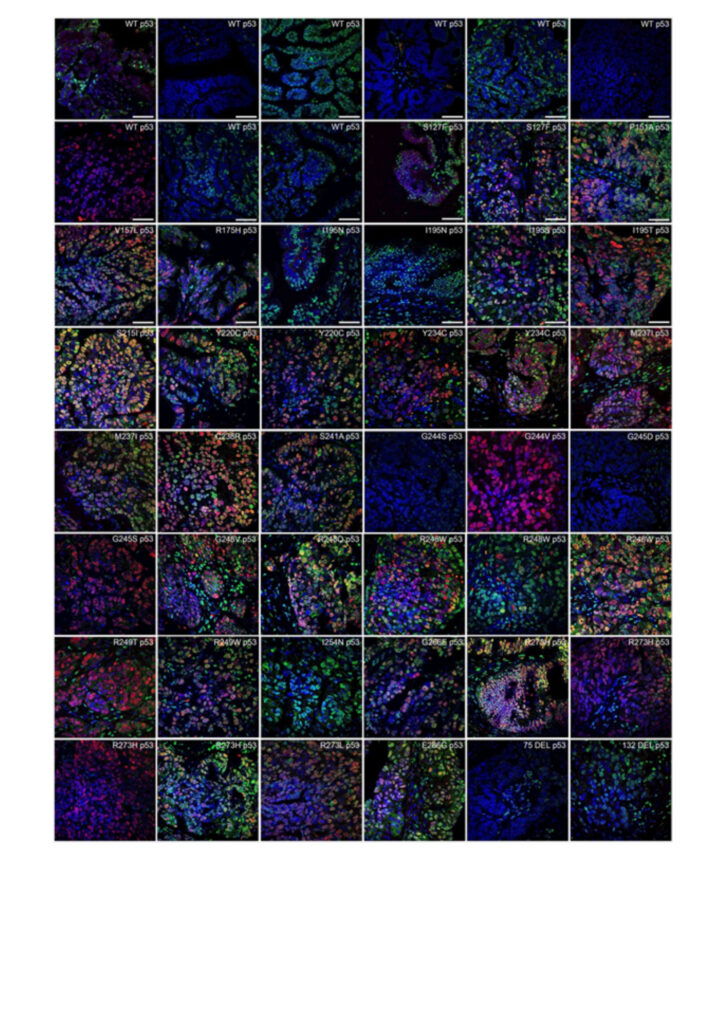
Method development for the detection of TP53 aggregates: In half of all cancers, TP53 is mutated and forms amyloid-like structures. In this study p53 aggregates were detected by co-immunofluorescence (Co-IF) in 38 of 78 (48.7%) FFPE tissue samples from OC patients. Figure 1 shows representative images from 48 patients. The positive wild-type (WT) samples as well as the varying degrees of p53 aggregation in patients with identical TP53 mutations suggest that a missense mutation alone is not sufficient to increase the ability of p53 to form aggregates.
The clinical relevance of p53 aggregates remains largely unknown, possibly due to the lack of sensitive and specific detection methods. Therefore, several methods for the detection of TP53 aggregates in OC were investigated (Co-IF, proximity ligation assay (PLA), co-immunoprecipitation (Co-IP), and p53-Seprion ELISA). PLA (for FFPE tissues) and p53-seprion ELISA (for FF tissues) were found to be the only two methods that allowed quantitative measurement of p53 aggregates. These methods could facilitate stratification of patients in future clinical trials targeting p53 aggregation.
Leader: Daniel Egle
MESI-STRAT collaboration: Collaboration with Prof. Dr. Thedieck (Institute of Biochemistry, Leopold-Franzens-University Innsbruck) within the EU project MESI-STRAT was initiated. MESI-STRAT develops metabolite marker panels measurable in biological fluids to enable the stratification of BC patients, resistance monitoring and clinical decision making during endocrine therapy (ET). This is a novel concept, as metabolism in BC has been inadequately studied for diagnostic and therapeutic purposes. In the prospective part of the study (WOO 2 -Trial), BC patients are recruited at the Dept. In the retrospective part (ET-Termination Trial), serum samples from the Dept.’s biobank from ET-treated BC patients at specific time points will be analysed.
Leader: Heidi Fiegl
Effect of methadone on chemotherapy in OC models: Methadone has been described in cell culture experiments as a chemo sensitizer in leukaemia and glioblastoma via the activation of opioid receptors. Several years ago, a real hype about methadone as a cancer drug arose, which also included patients from our Dept. Therefore, a study was conducted to investigate the effect of methadone in combination with cisplatin on different OC cell lines, but also on tumour spheroids derived from OC patients. Combined treatment showed no improvement in the therapeutic outcome as measured by a reduction in cell viability; in some cases, worse therapeutic response was observed. Based on these data, additional use of methadone as adjuvant support for chemotherapy in the treatment of OC cannot be recommended.
Identification of conserved subtypes of prognostic significance in cervical squamous cell carcinoma (CSCC): Integrated multiomics analyses of 643 CSCC tissues were performed as part of the collaboration with Prof. Dr. Tim Fenton (University of Southampton, UK). Two therapy-relevant CSCC subtypes (C1 and C2) with different prognosis were identified (independent of HPV type). Patients with C2 tumours (20% of CSCCs in the patient cohorts studied) had shorter survival, which may be explained by differential genomic alterations, increased expression of multiple immune checkpoint genes and differences in the tumour immunologic microenvironment.
Leader: Verena Wieser
Angiogenic tumour phenotype in OC: Blocking angiogenesis with bevacizumab, an antibody targeting vascular endothelial growth factor (VEGF), shows efficacy in different lines of OC therapy. This study investigated the clinical impact of expression of angiogenesis-related genes and their association with bevacizumab response in OC in a retrospective analysis of three independent cohorts. The study indicated that angiogenic gene expression in OC tumour tissues is associated with an unfavourable survival rate. The established score could be used to identify patients who respond to targeted antiangiogenic therapies.
Leader: Irina Tsibulak, Katharina Knoll
COVID-19 pandemic: A number of different studies (among others with the COVIDSurg Collaborative group) have evaluated the impact of the COVID-19 pandemic. A retrospective data analysis for 2020 compared to 2019 examined the effect of the COVID-19 pandemic on rates of newly diagnosed gynaecologic and breast cancers. As with many other diseases, both lockdowns led to a sharp decline in the number of diagnoses of these cancers, resulting in delays in treatment. Therefore, new strategies in patient care are needed to optimise cancer screening and treatment during challenging times such as a pandemic.
Leaders: Alain Zeimet, Irina Tsibulak
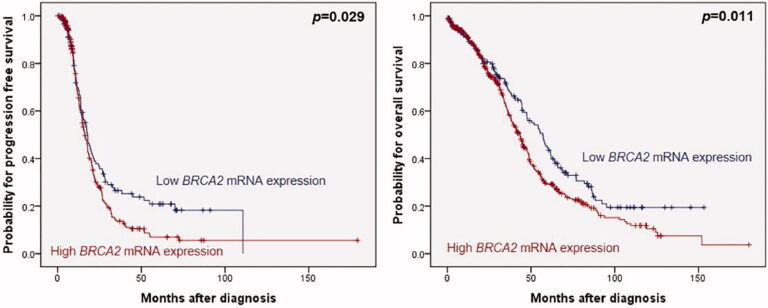
BRCA2 mRNA expression in OC: In previous studies the work group showed that low BRCA1 expression was associated with a favourable overall survival rate (OS) and low BRCA2 expression with better progression-free survival (PFS) and OS in BRCA WT cancers. In a validation study, the pathophysiologic impact of BRCA2 expression in HGSOC was underscored on the dataset from The Cancer Genome Atlas (TCGA) project (n = 581,
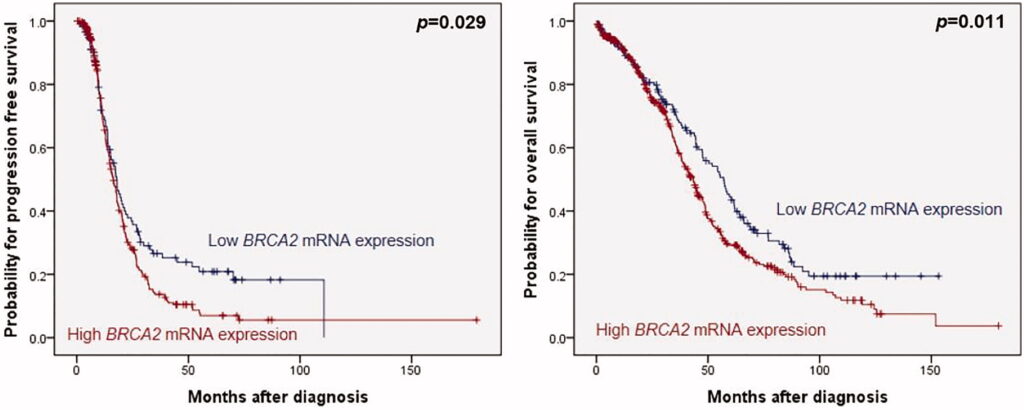
, but the clinical impact of BRCA1 expression was not confirmed.
Pregnancy Research:
Leader: Samira Abdel-Azim
Ophthalmic artery Doppler measurement: In a prospective observational study at 19 to 23 weeks’ gestation conducted at King’s College Hospital, London, UK, it was shown that the ophthalmic artery Doppler measurement of artery peak systolic velocity (PSV) ratio is likely to be useful in screening not only for hypertensive disorders of pregnancy, but also for pregnancies with a small-for-gestational-age (SGA) neonate.
Leader: Andreas Widschwendter, Samira Abdel-Azim
Influence of Pregnancy-Related Conditions on Human Epididymis Protein 4 (HE4) Serum Levels: It was shown that HE4 serum levels are affected by various pregnancy-related issues, resulting in significantly higher levels in these cases. Although medians varied by trimester, the 95th percentile cut-off and almost all maximum values throughout the course of pregnancy were below the established cut-off for premenopausal women. Consequently, HE4 can be used as a useful negative predictive marker for adnexal mass assessment during pregnancy.
Pictures
Selected Publications
- Abdel Azim, Samira; Wright, Alan; Sapantzoglou, Ioakeim; Nicolaides, Kypros. H.; Charakida, Marietta: Ophthalmic artery Doppler at 19-23 weeks’ gestation in pregnancies that deliver small-for-gestational-age neonates. ULTRASOUND IN OBSTETRICS & GYNECOLOGY. 2022; 60(1); 52-58.
PubMed: 35441758 doi: 10.1002/uog.24913 [FLDID: 134029]
- Chakravarthy, Ankur; Reddin, Ian; Henderson, Stephen; Dong, Cindy; Kirkwood, Nerissa; Jeyakumar, Maxmilan; Rodriguez, Daniela Rothschild; Martinez, Natalia Gonzalez; McDermott, Jacqueline; Su, Xiaoping; Egawa, Nagayasau; Fjeldbo, Christina S.; Skingen, Vilde Eide; Lyng, Heidi; Halle, Mari Kylleso; Krakstad, Camilla; Soleiman, Afschin; Sprung, Susanne; Lechner, Matt; Ellis, Peter J. I.; Wass, Mark; Michaelis, Martin; Fiegl, Heidi; Salvesen, Helga; Thomas, Gareth J.; Doorbar, John; Chester, Kerry; Feber, Andrew; Fenton, Tim R.: Integrated analysis of cervical squamous cell carcinoma cohorts from three continents reveals conserved subtypes of prognostic significance. NATURE COMMUNICATIONS. 2022; 13(1); 5818.
PubMed: 36207323 doi: 10.1038/s41467-022-33544-x [FLDID: 134893]
- Gnant, Michael; Fitzal, Florian; Rinnerthaler, Gabriel; Steger, Guenther G.; Greil-Ressler, Sigrun; Balic, Marija; Heck, Dietmar; Jakesz, Raimund; Thaler, Josef; Egle, Daniel; Manfreda, Diether; Bjelic-Radisic, Vesna; Wieder, Ursula; Singer, Christian F.; Melbinger-Zeinitzer, Elisabeth; Haslbauer, Ferdinand; Sevelda, Paul; Trapl, Harald; Wette, Viktor; Wimmer, Kerstin; Gampenrieder, Simon P.; Bartsch, Rupert; Kacerovsky-Strobl, Stephanie; Suppan, Christoph; Brunner, Christine; Deutschmann, Christine; Soelkner, Lidija; Fesl, Christian; Greil, Richard; Austrian Breast Co: Duration of Adjuvant Aromatase-Inhibitor Therapy in Postmenopausal Breast Cancer. NEW ENGLAND JOURNAL OF MEDICINE. 2021; 385(5); 395-405.
PubMed: 34320285 doi: 10.1056/NEJMoa2104162 [FLDID: 131273] IF: 176,082 / ZIT: 22
- Heinzl, Nicole; Koziel, Katarzyna; Maritschnegg, Elisabeth; Berger, Astrid; Pechriggl, Elisabeth; Fiegl, Heidi; Zeimet, Alain G.; Marth, Christian; Zeillinger, Robert; Concin, Nicole: A comparison of four technologies for detecting p53 aggregates in ovarian cancer. FRONTIERS IN ONCOLOGY. 2022; 12(S); 976725.
PubMed: 36158680 doi: 10.3389/fonc.2022.976725 [FLDID: 134493]
- Trutnovsky, Gerda; Reich, Olaf; Joura, Elmar A.; Holter, Magdalena; Ciresa-Koenig, Alexandra; Widschwendter, Andreas; Schauer, Christian; Bogner, Gerhard; Jan, Ziga; Boandl, Angelika; Kalteis, Martin S.; Regauer, Sigrid; Tamussino, Karl: Topical imiquimod versus surgery for vulvar intraepithelial neoplasia: a multicentre, randomised, phase 3, non-inferiority trial. LANCET. 2022; 399(10337); 1790-1798.
PubMed: 35483400 doi: 10.1016/S0140-6736(22)00469-X [FLDID: 134290]
Selection of Funding
- Comprehensive Testing of the functionality of Homologous Recombination (HR) in DNA Repair A step towards Personalised Treatment in “high-grade” Epithelial Ovarian Cancer – Astra Zeneca Research Funding
- Ovarian cancer treatment with Trabectedin and Lurbinectedin in a patient derived tumour spheroid model – Pharma Mar Research Funding
Collaborations
Collaborations in Clinical Trials with (inter)national research groups:
- AGO Austria – Arbeitsgemeinschaft Gynäkologische Onkologie
- ABCSG – Austrian Breast & Colorectal Cancer Study Group
- ENGOT – European Network for Gynecological Oncological Trial groups (Cooperation with 19 trial groups)
- GCIG – Gynecologic Cancer InterGroup (Cooperation with 25 international trial groups)
International collaboration with trial groups outside of ENGOT, GCIG or ABCSG trials:
- Dr. Jalid Sehouli, Charité Berlin, Germany; Nord-Ostdeutsche Gesellschaft für Gynäkologische Onkologie (NOGGO e.V.)
- EORTC – European Organisation for Research and Treatment of Cancer
Collaborations in Translational research projects:
- Dr. Kathrin Thedieck, Dept. of Biochemistry, University of Innsbruck, Innsbruck, Austria
- Dr. Robert Zeillinger, Medical University Vienna, Vienna, Austria
- Dr. Tim Fenton, Institute for Life Sciences, University of Southampton, Southampton, United Kingdom