Anichstraße 35
6020 Innsbruck
Fax: +43 512 504 23317
Email: guenter.weiss@i-med.ac.at
Website: http://Universitätsklinik für Innere Medizin II Innsbruck - Home (tirol-kliniken.at)/
Research Branch (ÖSTAT Classification)
302030, 301902, 302072, 303031
Keywords
anaemia research, Clinical and experimental infectious diseases, clinical immunology, diagnostic biomarkers, experimental and clinical pneumology, host-pathogen interaction, iron homeostasis, lipid metabolism, personalized medicine, and rheumatology
Research Focus
Our research focuses on the elucidation of the basic principles and clinical management of diseases at the bench and at the bedside, in order gain a detailed understanding of the biochemical, molecular and translational aspects of diseases, observed within the spectrum of our clinical expertise in internal medicine, infectious diseases, immunology, pneumology, rheumatology and general internal medicine.
General Facts
The department focuses on all clinical and scientific aspects of infectious diseases (ID), clinical immunology, pneumology and rheumatology.
On the clinical side, the department is a referral centre with special in-patient and outpatient facilities for ID, pneumology and rheumatology, and it is home to a diagnostic laboratory for ID and rheumatology. We also operate facilities for ultrasound, echocardiography, bronchoscopy and pulmonary function testing. Moreover, we cover all general aspects of internal medicine and appreciate the interconnections with all related medical disciplines.
On the scientific side, our research groups have a strong background in cellular and molecular biology as well as immunology, using state of the art analytical techniques. We have elucidated numerous mechanisms, diagnostic algorithms and novel therapeutic principles in a variety of diseases, including anaemia of inflammation, atherosclerosis, iron dyshomeostasis, infections with extracellular and intracellular microbes, host-pathogen interplay and the interconnections of iron, energy and lipid metabolism with the immune system. In our research laboratories and on our clinical wards, we investigate relevant topics in clinical ID, rheumatology and pneumology, using a wide range of up-to-date in silico, in vitro and in vivo technologies. In clinical research in ID, pneumology and rheumatology we have established registries and biobank sampling for common and rare diseases in these disciplines in order to better understand the specific course of various diseases in our fields of expertise, to identify clinical relevant disease pattern and novel biomarkers in order to established optimised therapies for our patients.
Research
The primary aim of our research groups is to translate molecular findings from clinical samples and disease models into better diagnostic and therapeutic tools, in order to improve the care of patients with infectious, inflammatory, metabolic, pulmonary and rheumatologic disorders. Given the strong background of our research groups in iron homeostasis, immunology, lipid metabolism and translational medicine, the focus of our combined research efforts is on the complex interplay of iron and cholesterol handling in infectious, inflammatory and metabolic diseases, on the pathophysiology and therapy of anaemia of inflammation (AI), on the improvement of rheumatologic disease classification and on the clinical management of patients with pulmonary diseases. The recent pandemic resulted in the focus on clinical and experimental research linked to SARS-COV2, which is based on expertise and previous studies on diseases pattern related to influenza and bacterial pneumonia.
Infectious Diseases – From Bacteria to Respiratory Viruses and the role of Nutritional Immunity
Infectious agents are combatted by the concerted action of innate and acquired immune mechanisms. A central feature of the innate immune response to pathogens, including viruses, bacteria and fungi, is the sequestration of nutrients such as iron and lipids. Collectively, the mechanism of nutrient withdrawal from microbes is termed “nutritional immunity”. Owing to its essential role for the growth and proliferation of microbes, iron is a major target of the nutritional immune response.
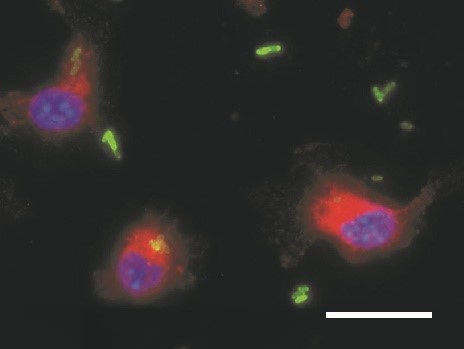
Disorders of iron homeostasis are both frequent and important to the clinical course and outcome of underlying and coexisting diseases. They are also relevant for global public health. For example, low-income countries have a particularly high prevalence of iron deficiency anaemia, in children as well as adults. However, region-wide oral iron supplementation in these countries has resulted in higher rates of severe infection, for reasons that remain largely unknown. We are interested in the impact of iron supplementation on the immune system and on the course of bacterial infections. We have observed that iron supplementation fuels the proliferation of Salmonella Typhimurium in macrophages (Fig. 1). We employed a mouse model mimicking iron deficiency anaemia and normal iron status and supplemented iron prior to the infection with Salmonella. These studies reveal that individuals with iron deficiency supplemented with iron have an impaired infection control and succumb to death faster. On one hand, this is due to increased iron availability for bacteria and related to reduced neutrophil function and CD8+ T- cell responses in iron deficient, anaemic mice on the other. Such studies have a major impact on preventive iron supplementation programmes in low-income countries.
We also want to understand how iron is compartmentalised in the host organism during infections. Specifically, different mechanisms exist to withhold iron from extracellular pathogens such as E. coli and Aspergillus fumigatus, as opposed to intracellular pathogens such as viruses, Chlamydia, Listeria or Salmonella species. The mechanisms involved need to be adapted and fine-tuned, depending on type and localisation of the noxious agent confronting the immune system. Delineating the signalling and metabolic pathways from pathogen or danger recognition to iron sequestration will allow us to characterise relevant mechanisms and to identify novel targets for therapeutic intervention.
In patients with sepsis, we found that elevated levels of the iron storage protein ferritin in plasma were associated with impaired survival. Specifically, we found positive correlations of increased plasma iron and ferritin concentration with mortality. Collectively, our results demonstrate that iron availability affects innate and adaptive immune responses to microbes and thus the course of infectious diseases.
Moreover, we have investigated multiple clinical aspects of respiratory viral and bacterial infections over many years, with a focus on influenza and pneumococcal/Legionella pneumonia. The emergence of the COVID-19 pandemic also directed our scientific interest towards aspects of this infection. We studied the association of anaemia and disturbances of iron homeostasis with course of COVID-19, we have validated and identified biomarkers and diagnostic tests for identification and disease prediction of COVID-19, and we have addressed organ involvement with a focus on the lung and the heart and the course of disease in patients with respiratory infections over time.
Iron, Lipid Homeostasis and Inflammatory Disorders
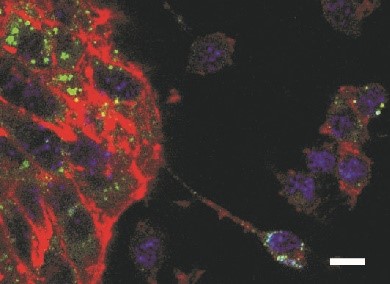
In our atherosclerosis and lipid metabolism research projects, we elucidated the interplay of iron and cholesterol homeostasis with innate immunity in a translational manner. By combining human genetic data with functional experiments, we identified an unexpected connection between iron homeostasis and cholesterol transport: we found that a single nucleotide polymorphism (SNP) in the haemochromatosis gene HFE is associated with reduced levels of atherogenic low-density lipoprotein (LDL) cholesterol in human plasma. We discovered that the underlying molecular mechanism is located in the liver, where HFE acts as a negative regulator of the LDL receptor. Kupffer cells act as gatekeepers of cholesterol homeostasis, as they accept LDL-derived cholesterol from plasma and transfer it to adjacent hepatocytes (Fig. 2) in a way that depends upon ATP-binding cassette transporter Abca1, which is regulated by iron disposal. In extending this study, we are in progress of developing novel therapeutics to modify lipid homeostasis and LDL receptor expression for the prevention and treatment of diseases associated with atherosclerosis.
In another project, we are investigating the alterations of lipid homeostasis and the impact of genetic lipid disorders including hypercholesterinaemia on the course of bacterial sepsis and its importance for innate and adaptive immune control. Our experiments indicate that both LDL and HDL contribute to innate immune response and modulate anti-microbial immune effector pathways.
A recent study in collaboration with the Department of Cardiac Surgery revealed a novel pathway whereby inflammatory signalling with Toll like receptor-3 is directly linked to the calcification of the aortic valve leading to eventual heart failure. This novel finding opens the door for new therapeutic interventions to treat this frequent clinical problem affecting primarily elderly by modulating the inflammatory cascade.
Finally, we are analysing immune metabolism in infectious and inflammatory diseases. We have previously shown that iron availability affects not only the innate immune response and macrophage polarisation, but also orchestrates T-helper cell differentiation via regulating the expression of T- cell immunoglobulin domain -3 (TIM-3). We are currently scrutinising the underlying molecular mechanisms, which are likewise contributing to impaired immune control of infections and malignant diseases. This leads to studies investigating the effects of immune cell polarisation by cytokines on immune effector functions in models of bacterial infection, and analyses of how amino acid metabolism, with a focus on arginine, tryptophan and tyrosine, affects innate immune responses and/or microbial survival. We are analysing the metabolic effects of iron on TCA- cycle activity and mitochondrial respiration in inflammatory conditions to gain further insights into the complex regulatory network of the immune metabolism in infections, which will have important implications for future therapeutic strategies (by modulating the inflammatory metabolic microenvironment, immune checkpoint regulation or cellular differentiation/activity/ survival).
Rheumatological Diseases
Rheumatological diseases are among the most prevalent of inflammatory disorders. We are actively involved with improving diagnosis and management of these diseases, also as part of national and international expert committees.
One of our aims is the international standardisation of autoantibody diagnosis, which is relevant for the classification of autoimmune and inflammatory disorders, including connective tissue diseases associated with antinuclear antibodies (ANA) and small-vessel vasculitides associated with anti-neutrophil cytoplasmic antibodies (ANCA). We work in close collaboration with the European Autoimmunity Standardisation Initiative (EASI) and with other members of the International Consensus on ANA Patterns (ICAP).
In large-vessel vasculitides and in arthritides, imaging is the key to initial diagnostic work-up, disease classification and the identification of patients at increased risk of severe course of disease. Together with other national and international experts, the department participates in the compilation of evidence-based guidelines for the diagnosis and treatment of giant cell arteritis. In ongoing clinical studies, we are extending our expertise in ultrasound of the musculoskeletal and vascular systems to other rheumatologic disorders, such as polymyalgia rheumatica, psoriatic arthritis and rheumatoid arthritis.
Furthermore, we are interested in the advancement of women’s careers in rheumatology, as females are especially prone to a “leaking pipeline” in both clinical and scientific rheumatology. In cooperation with specialists in the field of business and economics, we are assessing factors of the performance management practices of health care systems with impact on the “leaky pipeline”.
In addition to laboratory and ultrasound studies on rheumatologic diseases, one major research focus of the department is on one of its most frequent clinical consequences, i.e. anaemia of inflammation (AI).
Research in clinical rheumatology mainly focused on an extended view of quality of care. The research includes the perspective of probability in diagnoses and epidemiological studies in real-life data as performed in the context of comorbidities, polypharmacy and cardiovascular risks. Another aspect studied together with the European Alliance for Associations of Rheumatology (EULAR) was the role o nurses to optimise long-term management of the patients. Ultimately, an interventional study showed improvement of social and role functioning as proposed by the World Health Organisation (WHO). With more than 5000 orphan diseases involving the musculoskeletal system, an important quality focus is also the specific management of rare diseases such as i.e. Behcet’s disease.
Anaemia of Inflammation (AI)
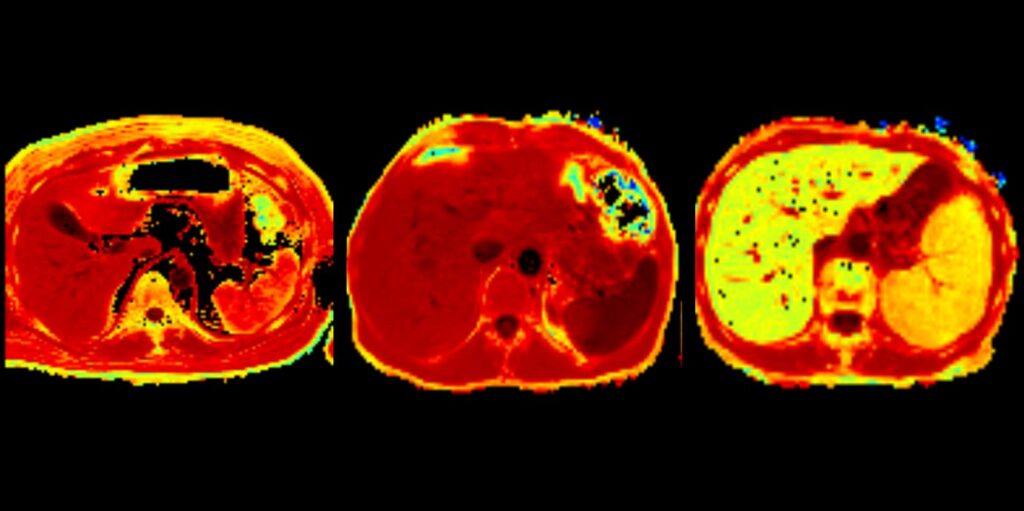
AI is a classic example of the connection between iron homeostasis and inflammation. Iron storage in macrophages is a major contributing factor in the pathogenesis of AI and renders iron unavailable for haemoglobin synthesis and red blood cell production. We have previously identified crucial pathways leading to AI mainly focusing on effects of cytokines on iron homeostasis in macrophages and by elucidating complex regulatory interaction of iron homeostasis with central innate immune effector pathways including the activity and formation of the iron hormone hepcidin. Therapeutic approaches to ameliorate anaemia by modifying hepcidin expression have been previously published by our laboratory. More recent studies systemically investigated the potential of oral and intravenous iron supplementation to treat AI. Although, these therapies are widely used in clinical practice, they have been insufficiently studied in subjects with inflammatory diseases and anaemia. We found that intravenous and oral iron are ineffective to induce erythropoiesis and to correct anaemia in case of advanced inflammation. Of note, intravenous iron was retained in the spleen and liver and promoted the formation of inflammatory cytokines. However, when AI was associated with true or absolute iron deficiency on the basis of concomitant bleeding, both oral and intravenous iron ameliorated haemoglobin levels and anaemia. This pointed to the importance of the proper differentiation between AI and AI with true iron deficiency (AI+IDA). Based on the long-lasting expertise at the department of radiology in Innsbruck (B. Henninger and his group), we investigated organ iron stores in subjects with AI and AI+IDA and compared the results to established serum markers of iron homeostasis and erythropoiesis. This study revealed that AI resulted in tissue iron retention, whereas AI +IDA was associated with low iron levels in the spleen liver and heart, indicating putative response to iron supplementation therapy. We have also studied AI and its associated to disease severity and outcome in different important diseases such as congestive heart failure, chronic pulmonary diseases and infections including COVID-19 and influenza.
Together, our studies continue to identify links between inflammation and iron homeostasis and disease outcome, for a better understanding of pathophysiological mechanisms and an improved diagnosis and targeted therapy of patients with inflammatory anaemia.
Pulmonary Diseases Including Sequelae of COVID-19
The department conducts long-term studies on pneumonia, chronic obstructive pulmonary disease (COPD), asthma, interstitial lung disease (ILD) and pulmonary arterial hypertension (PAH). In this context, academic patient registries and biobanks have been established in order to identify phenotypes, comorbidity-clusters and biomarkers. In particular, we revealed that machine-learning approaches may improve risk management and harness clinical variability. Thereby, supervised and unsupervised learning algorithms, such as Elastic Net regression and medoid clustering, are powerful tools for automated mortality risk prediction in PAH. Especially for this patient group, therapy escalation and early access to trials and new drug strategies is important, as is the case in our industry-sponsored international trials. For patients with systemic sclerosis, we are investigating a better risk prediction for both PAH and pulmonary fibrosis, in close collaboration with other disciplines.
The COVID-19 pandemic, we established and validated diagnostic and therapeutic strategies and explored predictors for severe disease courses, such as pneumonia and hyper-inflammation syndrome as well as delayed cardiopulmonary recovery. In the post-pandemic phase, we continue to be challenged with the long-term consequences of SARS-CoV-2 infection. Broad interdisciplinary coordination has enabled the establishment of care pathways as well as prospective registries to better characterise long-term outcomes. We established the prospective “CovILD trial” for a systematic cardiopulmonary follow-up of patients post COVID-19 and reported that elevated plasma ferritin levels and AI are associated with the severity of COVID-19.
At 12 months after the SARS-CoV-2 infection, persistent symptoms continued to be found in 65% of study participants; 33% suffered from lung function impairment; 51% showed CT abnormalities; and 63% had low-grade diastolic dysfunction. Main risk factors for persisting cardiopulmonary impairment included increased levels of pro-inflammatory and immunological biomarkers at early visits. In addition, we deciphered three recovery clusters distinguishing almost complete recovery in patients with post-acute inflammatory profiles and enrichment in cardiopulmonary residuals in a female-dominated post-COVID-19 syndrome with reduced mental health status.
We have also initiated an interdisciplinary prospective registry to characterise non-hospitalised SARS-CoV-2 infected individuals with persisting symptoms (PRECISE registry: Prospective Multidisciplinary Post-COVID-19 Registry Tyrol). First analyses have contributed to an improved characterization of the post-COVID condition.
Summary
The overlap between the medical specialisations in the department creates tremendous synergies. Our strong clinical and scientific background enables us to combine basic and clinical research and to gain new knowledge in both areas. Our aim is to personalise and optimise diagnostic tools, to refine the classification of diseases and the prognostic assessment of patients, and to improve treatment strategies in infectious diseases, clinical immunology, pneumology and rheumatology.
Pictures
Selected Publications
Infectious/Inflammatory Diseases and Immune-metabolism
- Hoffmann A, Haschka D, Valente de Souza L, Tymoszuk P, Seifert M, von Raffay L, Hilbe R, Petzer V, Moser PL, Nairz M, Weiss G. Baseline iron status and presence of anaemia determine the course of systemic Salmonella infection following oral iron supplementation in mice. EBioMedicine. 2021 Sep;71:103568. doi: 10.1016/j.ebiom.2021.103568. Epub 2021 Sep 3.PMID: 34488018
- Gollmann-Tepeköylü C, Graber M, Hirsch J, Mair S, Naschberger A, Pölzl L, Nägele F, Kirchmair E, Degenhart G, Demetz E, Hilbe R, Chen HY, Engert JC, Böhm A, Franz N, Lobenwein D, Lener D, Fuchs C, Weihs A, Töchterle S, Vogel GF, Schweiger V, Eder J, Pietschmann P, Seifert M, Kronenberg F, Coassin S, Blumer M, Hackl H, Meyer D, Feuchtner G, Kirchmair R, Troppmair J, Krane M, Weiss G, Tsimikas S, Thanassoulis G, Grimm M, Rupp B, Huber LA, Zhang SY, Casanova JL, Tancevski I, Holfeld J.Toll-Like Receptor 3 Mediates Aortic Stenosis Through a Conserved Mechanism of Calcification. 2023 May 16;147(20):1518-1533. doi: 10.1161/CIRCULATIONAHA.122.063481. Epub 2023 Apr 4.PMID: 37013819
- Brigo N, Neumaier E, Pfeifhofer-Obermair C, Grubwieser P, Engl S, Berger S, Seifert M, Reinstadler V, Oberacher H, Weiss G.Timing of Interleukin-4 Stimulation of Macrophages Determines Their Anti-Microbial Activity during Infection with Salmonella enterica Serovar Typhimurium. Cells. 2023 Apr 14;12(8):1164. doi: 10.3390/cells12081164.
- Thommes L, Burkert FR, Öttl KW, Goldin D, Loacker L, Lanser L, Griesmacher A, Theurl I, Weiss G, Bellmann-Weiler R. Comparative evaluation of four SARS-CoV-2 antigen tests in hospitalized patientsInt J Infect Dis. 2021 Apr;105:144-146. doi: 10.1016/j.ijid.2021.02.052. Epub 2021 Feb 17.PMID: 33609774
- Haschka D, Tymoszuk P, Petzer V, Hilbe R, Heeke S, Dichtl S, Skvortsov S, Demetz E, Berger S, Seifert M, Mitterstiller AM, Moser P, Bumann D, Nairz M, Theurl I, Weiss G. Ferritin H deficiency deteriorates cellular iron handling and worsens Salmonella typhimurium infection by triggering hyperinflammation. JCI Insight. 2021 Jul 8;6(13):e141760. doi: 10.1172/jci.insight.141760. PMID: 34236052
Iron Homeostasis, Metabolism and Anemia Research
- Valente De Souza L, Hoffmann A, Fischer C, Petzer V, Asshoff M, Theurl I, Tymoszuk P, Seifert M, Brigo N, Hilbe R, Demetz E, Von Raffay L, Berger S, Barros-Pinkelnig M, Weiss G. Comparative analysis of oral and intravenous iron therapy in rat models of inflammatory anemia and iron deficiency. Haematologica. 2023 Jan 1;108(1):135-149. doi:10.3324/haematol.2022.281149.PMID: 35796011
- Lanser L, Burkert FR, Bellmann-Weiler R, Schroll A, Wildner S, Fritsche G, Weiss G. Metabolites. Dynamics in Anemia Development and Dysregulation of Iron Homeostasis in Hospitalized Patients with COVID-19. 2021 Sep 25;11(10):653. doi: 10.3390/metabo11100653.PMID: 34677368
- Fischer C, Valente de Souza L, Komlódi T, Garcia-Souza LF, Volani C, Tymoszuk P, Demetz E, Seifert M, Auer K, Hilbe R, Brigo N, Petzer V, Asshoff M, Gnaiger E, Weiss G. Mitochondrial Respiration in Response to Iron Deficiency Anemia: Comparison of Peripheral Blood Mononuclear Cells and Liver. Metabolites. 2022 Mar 21;12(3):270. doi: 10.3390/metabo12030270.PMID: 35323713
- Sonnweber T, Grubwieser P, Sahanic S, Böhm AK, Pizzini A, Luger A, Schwabl C, Koppelstätter S, Kurz K, Puchner B, Sperner-Unterweger B, Hüfner K, Wöll E, Nairz M, Widmann G, Tancevski I, Löffler-Ragg J, Weiss G. The Impact of Iron Dyshomeostasis and Anaemia on Long-Term Pulmonary Recovery and Persisting Symptom Burden after COVID-19: A Prospective Observational Cohort Study. Metabolites. 2022 Jun 14;12(6):546. doi: 10.3390/metabo12060546.PMID: 35736479
- Lanser L, Plaikner M, Schroll A, Burkert FR, Seiwald S, Fauser J, Petzer V, Bellmann-Weiler R, Fritsche G, Tancevski I, Duftner C, Pircher A, Seeber A, Zoller H, Kremser C, Henninger B, Weiss G. Tissue iron distribution in patients with anemia of inflammation: Results of a pilot study. Am J Hematol. 2023 Jun;98(6):890-899. doi: 10.1002/ajh.26909. Epub 2023 Mar 15.PMID: 36880875
Rheumatology
- von Mühlen CA, Garcia-De La Torre I, Infantino M, Damoiseaux J, Andrade LEC, Carballo OG, Conrad K, Francescantonio PLC, Fritzler MJ, Herold M, Klotz W, de Melo Cruvinel W, Mimori T, Satoh M, Musset L, Chan EKL. How to report the antinuclear antibodies (anti-cell antibodies) test on HEp-2 cells: guidelines from the ICAP initiative. Immunol Res. 2021 Dec;69(6):594-608. doi: 10.1007/s12026-021-09233-0. Epub 2021 Oct 9.PMID: 34625914
- DejacoC,KerschbaumerA,AletahaD,BondM,HysaE,CamellinoD,EhlersL,AbrilA,AppenzellerS,CidMC,DasguptaB,DuftnerC,GraysonPC,HellmichB,HočevarA,KermaniTA,MattesonEL,MollanSP,NeillLP,PonteC,SalvaraniC,SattuiSE,SchmidtWA,SeoP,SmolenJS,ThielJ,Toro-GutiérrezCE,WhitlockM,ButtgereitF. Treat-to-targetrecommendations in giant cell arteritis and polymyalgia rheumatica. Ann Rheum Dis 2023, Feb 24;ard-2022-223429. doi: 10.1136/ard-2022-223429.
- Sautner J, Grabner I, Posch A,Duftner C.How to plug the leaky pipeline in clinical rheumatology across Europe-lessons to be learned from experiences in business. Rheumatology (Oxford). 2023 Mar 1:kead090. doi: 10.1093/rheumatology/kead090.
- Karnik J, Riedl D, Schirmer M(2023) Improved social functioning and role functioning in rheumatic patients using a non-verbal communication tool: Results from a randomized, double-blind, controlled pilot-study Frontiers in Medicine 10: 684.
- Yagensky V, Michael Schirmer (2022) Cardiovascular Risks and Risk Stratification in Inflammatory Joint Diseases: A Cross-Sectional Study. Frontiers in medicine 9: 02.
Pneumology
- Sonnweber T, Tymoszuk P, Sahanic S, Boehm A, Pizzini A, Luger A, Schwabl C, Nairz M, Grubwieser P, Kurz K, Koppelstätter S, Aichner M, Puchner B, Egger A, Hoermann G, Wöll E, Weiss G, Widmann G, Tancevski I, Löffler-Ragg J. Investigating phenotypes of pulmonary COVID-19 recovery: A longitudinal observational prospective multicenter trial. Elife. 2022 Feb 8;11:e72500. doi: 10.7554/eLife.72500. PMID: 35131031
- Sahanic S, Tymoszuk P, Ausserhofer D, Rass V, Pizzini A, Nordmeyer G, Hüfner K, Kurz K, Weber PM, Sonnweber T, Boehm A, Aichner M, Cima K, Boeckle B, Holzner B, Rumpold G, Puelacher C, Kiechl S, Huber A, Wiedermann CJ, Sperner-Unterweger B, Tancevski I, Bellmann-Weiler R, Bachler H, Piccoliori G, Helbok R, Weiss G, Loeffler-Ragg J. Phenotyping of acute and persistent COVID-19 features in the outpatient setting: exploratory analysis of an international cross-sectional online survey. Clin Infect Dis. 2022 Nov 26: ciab 978. doi:10.1093/cid/ciab978. Online ahead of print. PMID: 34849652
- Luger AK, Sonnweber T, Gruber L, Schwabl C, Cima K, Tymoszuk P, Gerstner AK, Pizzini A, Sahanic S, Boehm A, Coen M, Strolz CJ, Wöll E, Weiss G, Kirchmair R, Feuchtner GM, Prosch H, Tancevski I, Löffler-Ragg J, Widmann G. Chest CT of Lung Injury 1 Year after COVID-19 Pneumonia: The CovILD Study. Radiology. 2022 Mar 29:211670. doi: 10.1148/radiol.211670. Online ahead of print. PMID: 35348379
- Sonnweber T, Grubwieser P, Pizzini A, Boehm A, Sahanic S, Luger A, Schwabl C, Widmann G, Egger A, Hoermann G, Wöll E, Puchner B, Kaser S, Theurl I, Nairz M, Tymoszuk P, Weiss G, Joannidis M, Löffler-Ragg J, Tancevski I. Pulmonary recovery from COVID-19 in patients with metabolic diseases: a longitudinal prospective cohort study. Sci Rep. 2023 Feb 14;13(1):2599. doi: 10.1038/s41598-023-29654-1.
- Nairz M, Sahanic S, Pizzini A, Böhm A, Tymoszuk P, Mitterstiller AM, von Raffay L, Grubwieser P, Bellmann-Weiler R, Koppelstätter S, Schroll A, Haschka D, Zimmermann M, Blunder S, Trattnig K, Naschberger H, Klotz W, Theurl I, Petzer V, Gehrer C, Mindur JE, Luger A, Schwabl C, Widmann G, Weiss G, Löffler-Ragg J, Tancevski I, Sonnweber T. Quantity of IgG response to SARS-CoV-2 spike glycoprotein predicts pulmonary recovery from COVID-19. Sci Rep. 2022 Mar 7;12(1):3677. doi: 10.1038/s41598-022-07489-6. PMID: 35256646
Selection of Funding
- Christian Doppler Society, Christian Doppler Laboratory for Iron Metabolism and Anemia research, Günter Weiss
- Austrian Research Funds (FWF)-Doctoral Programme (HOROS—W-1253), Günter Weiss
- Austrian Reseach Funds (FWF) Doc-Fund project-82 (Cellular Basis of Disease), Günter Weiss
- ERA-INFECT –FWF Joint Project (I-3321) , Günter Weiss
- ACOVACT (Austrian Ministry of Science), Günter Weiss
- Investigator-Initiated Study (IIS) grant by Boehringer Ingelheim (IIS 1199-0424), Ivan Tancevski
- Austrian Research Funds (FWF) Project P 33062, Manfred Nairz
- Tyrolean research Funds- Long Covid, Katharina Kurz/Judith Löffler-Ragg
- MUI Start Project- David Haschka
- ÖGR Research Grant- Lukas Lanser
Collaborations
- Sarah H. Atkinson, Kenya Medical Research Institute (KEMRI) Centre for Geographic Medicine Coast, KEMRI-Wellcome Trust Research Programme, Kilifi, Kenya
- Simone Appenzeller, Department of Orthopedics, Rheumatology and Traumatology (DORT),School of Medical Science, University of Campinas (UNICAMP), Brasil
- Christian Bogdan, Institute of Clinical Microbiology, Immunology and Hygiene, Friedrich-Alexander-Universität Erlangen-Nürnberg, Erlangen, Germany
- Dirk Bumann, Biocenter, Basel, Schweiz
- Jean-Laurent Casanova, Lab of Genetics of Human Infectious Diseases, Rockefeller University, New York, USA
- Christian Dejaco, Department of Rheumatology, Hospital of Brunico (SABES-ASDAA), Bruneck, Italy
- Isabella Grabner, Institute for Strategy and Managerial Accounting, Vienna University of Economics and Business Administration, Vienna, Austria
- Jonathan Jantsch, Inst. Für Med. Mikrobiologie, Univ. Köln, D
- Martina U. Muckenthaler, Department of Pediatric Hematology, Oncology and Immunology, University of Heidelberg, Heidelberg, Germany
- Miguel Soares, Institute Gulbenkain di Science, Lisboa, Portugal