Anichstraße 35
6020 Innsbruck
Fax: +43 9003 23144
Email: Joachim.schmutzhard@i-med.ac.at
Website: https://hno.tirol-kliniken.at/
Research Branch (ÖSTAT Classification)
3011, 3013, 3014, 106013, 106018, 106023, 106037, 106049, 106052, 106057, 302023, 302027, 302029, 302055, 302056, 302080, 302092
Keywords
clinical outcomes research, computer aided surgery, epithelial-mesenchymal transition, head and neck cancer, inner ear, neurobionic interface, oncolytic viruses, Otorhinolaryngology, radiomics, and spatial gene expression analysis and patient derived disease models.
Research Focus
Diagnosis, decision-making, innovative treatments, precision medicine and quantitative state-of-the art treatment outcome. Tumor biology supports clinical management of patients via predicting therapy resistance. Tumor-stroma interaction, metabolic modifiers of tumor immune system, and immunotherapy support clinical decisions. Artificial intelligence approaches support clinical decisions. Inner ear research focuses on hearing including computer simulations of neural hearing and equilibrium.
General Facts
The University Hospital for Otorhinolaryngology provides healthcare for the greater Tyrolean area. It has 3 operating theatres, 3 wards with 41 beds and an outpatient clinic. On average, 2.800 inpatients and 25.000 outpatients are treated every year. In addition to all standard otorhinolaryngologic surgical procedures, we provide cochlear implants (Fig. 1),
hypoglossal implants, sialendoscopy and Eustachian tube dilation. The state-of-the-art technical facilities include intraoperative navigation, neuro-monitoring and various laser types such as CO2, KTP and Erbium laser.
We have 3 basic clinical research laboratories, a clinical head & neck cancer registry (over 1.200 entries), a chronic rhinosinusitis patient registry (~ 300 patients), and a scientific biobank (-190 °C, samples of tumours (230), diseased paranasal sinus mucosa (100) and healthy control samples (80)). FFPE: 800 tumour and 300 paranasal sinus samples; RNA isolates of 80 tumours and 12 controls with checked gene expression.
Research
Inner Ear
R. Glückert, A. Schrott-Fischer
Human inner ear development and congenital abnormalities as well as auditory nerve regeneration are the main research focus. Basic research on age-related hearing loss and finding improvements for electrical stimulation of the auditory and vestibular nerve with organ explant and cellular models as well as computer simulation based on high-resolution 3D datasets bridge our research to cochlear implant companies. Comparative analyses of several mammalian animal models with the human inner ear complete our portfolio for translational inner ear research.
- Computer Simulation of Electrical Stimulation:
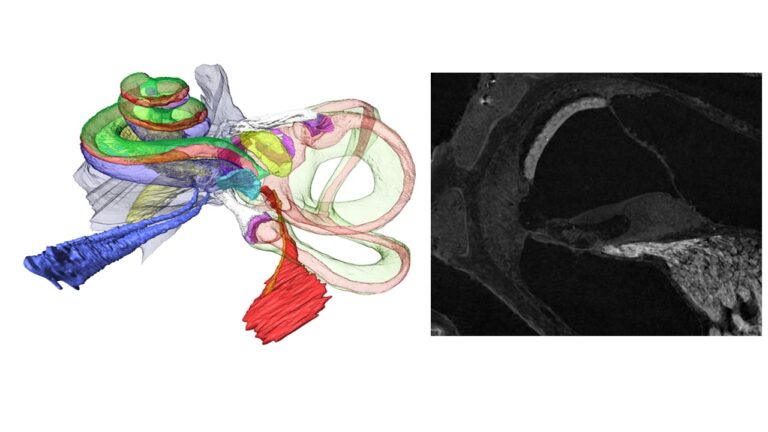
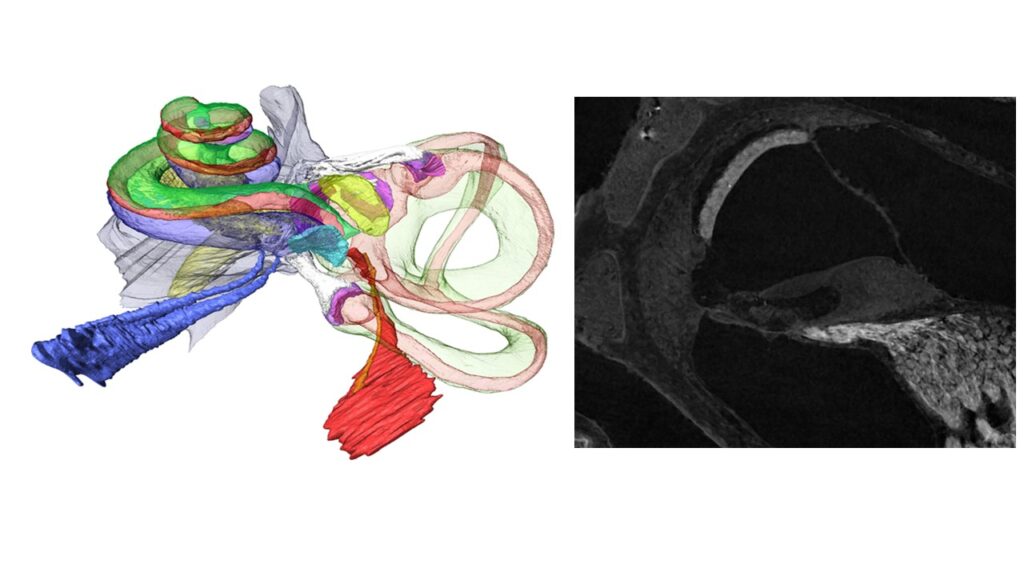
advanced correlative imaging from high-resolution microCT down to electron microscopy provides morphometric data, automated immune-histochemistry and image analysis of functional aspects for cellular and finite element modelling, Figure 2.
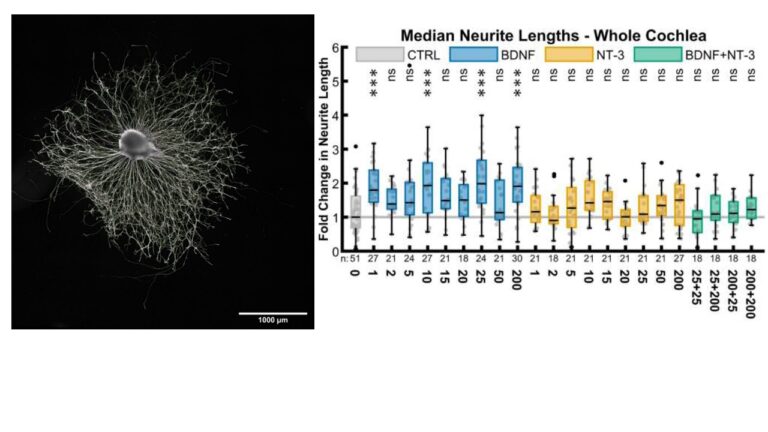
- Regeneration of the cochlear nerve for a gapless man:machine cochlear implant interface: after hair cell loss, auditory neurons survive for decades as monopolar amputated neurons. Regeneration of the peripheral axons and growth towards a cochlear implant could increase stimulations specificity and boost speech understanding. Neurotrophic factors are able to stimulate this outgrowth as we showed in in vivo and in vitro experiments, Figure 3.
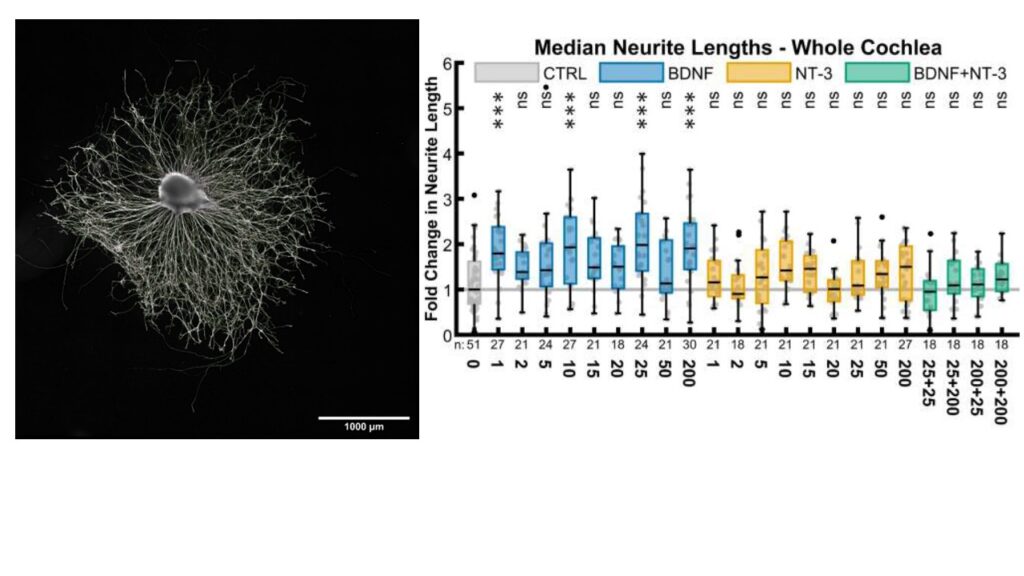
- Inner Ear Development:
to understand basic mechanisms of inner ear regeneration and signalling pathways knowledge about the cell fate and differentiation during embryology and foetal development is important. Especially the function of neurotrophin brain-derived neurotrophic factor (BDNF), its receptors and associated factors in developing human inner ear is a current research focus.
Radiomics and Artificial Intelligence
Z. Bardosi, D. Dejaco, W. Freysinger, Y. Özbek, M. Regodic, H. Riechelmann, M. Santer
The main research of this group focuses on providing tools to surgeons to aid intraoperative orientation and decisions in microscopic surgery in general.
While the surgical placement of auditory brain stem implants has become a routine intervention there is still ample space for improving the human computer interaction in surgery. A joint MedEl and FFG sponsored project demonstrated that intuitive computer interfaces for surgeons can be of significant value in such procedures. The paradigms of acoustic and visual guidance were developed and evaluated in a preclinical setting, respectively. The tools received very satisfactory feedback from surgeons and may eventually provide a roadmap for further exploitation.
In addition to this, another effort is to identify anatomical structures in the live stereo-microscopic view of the surgical site, ultimately in real-time and autonomously. Combining state-of-the-art intraoperative navigation technology with maximum application accuracy and deep learning based system calibration and identification of anatomical structures pushes the current limitations of navigation and knowledge / information support in the petrous bone and beyond.
Machine learning as a clinical decision support tool is a new technology to assist in classifying clinical decision pathways. This project’s goal is to find the most relevant features in diagnostic CT imagery of ENT tumour patients to identify potential non-responders or provide hints for an optimised clinical treatment comprising surgery, radio- and chemotherapy or combinations thereof.
Tumour biology
J. Dudas, J. Ingruber, M do Carmo Greier, H. Riechelmann, A. Runge, V. Schartinger, T. Steinbichler,
This group focuses on supporting the clinical management of head and neck cancer patients by determining predictive factors for therapy resistance, investigation of the mechanisms of resistance and proposing palliative therapy to overcome the resistance. Investigations of tumour-stroma interaction and metabolic modifiers of tumour immune system and immunotherapy explore new clinical routes. Molecular pathways of neurotrophin regulation of the developing human inner ear are explored in the context of ENT cancers.
- Neurotrophins in the developing inner ear and in HNSCC: The project investigates the quantitative gene expression changes of neurotrophin BDNF and other neurotrophins at mRNA level using real-time PCR and next generation sequencing RNASeq in the developing human inner ear. BDNF-associated factors and BDNF receptors are co-analysed in the bioinformatics follow-up of the RNASeq data. Gene ontology and interactive prediction models as KEGG- and WIKIpathways allow investigation of the co-expression and bio-mechanistic patterns of neurotrophins and associated genes in the timeline of human cochlea development.
- Predictive markers of immune checkpoint therapy focusses on the loss of HLA class I expression in melanoma and head and neck squamous carcinoma, as potential predictive factors for inefficiency of immune checkpoint inhibitor therapy. Downregulation of HLA class I expression was found mainly in progressing lesions of nonresponding patients; in contrast, minimal or no change in HLA class I expression was found in responding patients.
- Permissivity and mode of action of oncolytic viruses in ex vivo samples and slice cultures of human head and neck cancer studies in a preclinical environment a live recombinant oncolytic virus with an interferon-dependent tumour specificity induced oncolysis and stimulation of antitumor immune activity in permissive solid tumours without relevant neurotoxicity. In addition to providing patients-derived research material for VSV-GP permissivity studies by ViraT, the Molecular Oncology Lab of the ENT-clinic Innsbruck focusses on the topic of the response of tumour-infiltrating immune cells to the metabolic stress conditions.
- Exosomes in Head and Neck Squamous Cell Carcinoma are extracellular vesicles that might deliver components for resistance to standard therapy in head and neck cancer. Their cargo contains proteins of signalling pathways, RNAs and microRNA, and even double-stranded DNA. We hypothesise that EMC-exosomes are major objects in disseminating therapy resistance in HNC.
- Targeting of factors responsible for therapy resistance in head and neck cancer: scattered, clustered and diffuse detection of Slug in a pre-therapy biopsy predicts radio-chemotherapy resistance allowing up-front surgery as possible first-line therapy. The stabilised slug achieves protein interaction-based support of DNA-damage repair, which is unrelated to its transcription repressor activity. Our further research focusses on Slug-stabilisers and suppression of Slug by microRNA mimics or inhibitors in order to offer effective therapy extension to up-front surgery for Slug-positive HNC patients.
Further research projects include peripheral arterial tonometry for the investigation of paediatric sleep apnoea, effects of head & neck cancer treatment on intestinal microbiome and cachexia, development and validation of instruments to assess clinical outcomes, and the role of viral infections in the pathogenesis of middle ear disease and head & neck cancer.
Pictures
Selected Publications
Publications working group “Tumor Biology” lead by Dudas
- M do Carmo Greier, A. Runge, J. Dudas, V. Pider, I.-I. Skvortsova, D. Savic, H. Riechelmann
Mitochondrial dysfunction and epithelial to mesenchymal transition in head neck cancer cell lines.
Scientific Reports 12(1), 13255 (2022). - J. Ingruber, J. Dudas, D. Savic, G. Schweigl, M. Santer, S. Carollo, Z. Trajanoski, H. Riechelmann, T. Steinbichler, M. do Carmelo Greier
EMT-related transcription factors and protein stabilization mechanisms involvement in cadherin switch of head and neck squamous cell carcinoma.
Exp. Cell. Res. 414(1), 113084 (2022). - J. Ingruber, J. Dudas, S. Sprung, B. Lungu, F. Mungenast
Interplay between Partial EMT and Cisplatin Resistance as the Drivers for Recurrence in HNSCC.
Biomedicines 10(10), 2482 (2022). - A. Ladanyi, B. Hegyi, T. Balatoni, G. Liszkay, R. Rohregger, C. Waldnig, J. Dudas, S. Ferrone
HLA Class I Downregulation in Progressing Metastases of Melanoma Patients Treated With Ipilimumab.
Pathol Oncol Res 28(S), 1610297 (2022). - H. Riechelmann, T. Steinbichler, S. Sprung, M. Santer, A. Runge, U. Ganswindt, G. Gamerith, J. Dudas
The Epithelial-Mesenchymal Transcription Factor Slug Predicts Survival Benefit of Up-Front Surgery in Head and Neck Cancer.
Cancers 13(4), 772 (2021).
Publications working group “Radiomics and Artificial Intelligence” lead by Freysinger
- M. Berger, M. Pillei, A. Giotakis, A. Mehrle, W. Recheis, F. Kral, M. Kraxner, H. Riechelmann, W. Freysinger
Pre-surgery planning tool for estimation of resection volume to improve nasal breathing based on Lattice Boltzmann fluid flow simulations.
Int. J. Computer Assisted Radiology and Surgery, 16, 567 – 578 (2021). - M. Regodic, Z. Bardosi, W. Freysinger
Automated fiducial marker detection and localization in volumetric CT images: a three-step hybrid approach with deep learning.
J. Med. Imag. 8(2), 025002-1 – 025002-19 (2021). - Regodic, C. F. Freyschlag, J. Kerschbaumer, M. Galijasevic, W. Freysinger
Novel microscope-based visual display and nasopharyngeal registration for auditory brainstem implantation: a feasibility study in an ex vivo model.
Int. J. Computer Assisted Radiology and Surgery, 17, 261 – 270 (2022). - Santer, M. Kloppenburg, T. M. Gottfried, A. Runge, J. Schmutzhard, S. M. Vorbach, J. Mangesius, D. Riedl, S. Mangesius, G. Widmann, H. Riechelmann, D. Dejaco, W. Freysinger
Current applications of Artificial Intelligence to classify lymph nodes in patients with Head and Neck Squamous Cell Carcinom – a systematic review.
Cancers 14, 5397, 1 – 19 (2022). - R. Bardosi, D. Dejaco, M. Santer, M. Kloppenburg, S. Mangesius, G. Widmann, U. Ganswindt, G. Rumpold, H. Riechelmann, W Freysinger
Benchmarking eliminative radiomic feature selection for head and neck lymph node classification.
Cancers, 14, 477 (2022)
Publications working group “Inner Ear” lead by Schrott-Fischer and Glückert
- Schmidbauer, S. Fink, F. Rousset, H. Lowenheim, P. Senn, R. Glueckert
Closing the Gap between the Auditory Nerve and Cochlear Implant Electrodes: Which Neurotrophin Cocktail Performs Best for Axonal Outgrowth and Is Electrical Stimulation Beneficial?
Int J Mol Sci. 24(3) (2022). - M. Croner, A. Heshmat, A. Schrott-Fischer, R. Glueckert, W. Hemmert, S. Bai
Effects of Degrees of Degeneration on the Electrical Excitation of Human Spiral Ganglion Neurons Based on a High-Resolution Computer Model.
Frontiers in neuroscience 16:914876 (2022). - Hausott, R. Glueckert, A. Schrott-Fischer, L. Klimaschewski
Signal Transduction Regulators in Axonal Regeneration.
Cells 11(9) (2022). - Steinacher, L. J. Chacko, W. Liu, H. Rask-Andersen, W. Bader, J. Dudas, C. M. Sergi, T. Dhanaseelan, N. Moreno, R. Glueckert, R. Hoermann, A. Schrott-Fischer
Visualization of macrophage subsets in the development of the fetal human inner ear.
Front Immunol 13, 965196 (2022). - Heshmat, S. Sajedi, A. Schrott-Fischer, F. Rattay
Polarity Sensitivity of Human Auditory Nerve Fibers Based on Pulse Shape, Cochlear Implant Stimulation Strategy and Array.
Frontiers in Neuroscience 15, 751599 (2021).
Selection of Funding
- Dudas, FWF, Predictive Markers of Immune Checkpoint Inhibitor Therapy, 50 k€.
- Dudas, FWF, Neurotrophins in inner ear and head and neck cancer, 250 k€
- Dudas, Vira Therapeutics, Permissivity and mode of action of oncolytic viruses in ex vivo slice cultures of human head and neck cancer, 260 k€.
- Dudas, EU – COST, IMMUNO-model – Modelling immunotherapy response and toxicity in cancer.
- Freysinger, FWF, doc.funds IGDT-ART, sub-project 8, 200 k€.
- Freysinger, FWF, doc.funds IGDT-ART, sub-project 12, 200 k€.
- Glückert, FWF -DACH, GAPLESS Man:Machine Interface for the Inner ear, 300 k€.
- Glückert, MedEl, Evaluation of Structure Preservation in Implanted Temporal Bones for Vestibular Implant Electrode Design, 60 k€.
- Schrott-Fischer, FWF-DACH, Electric simulation of human hearing nerves – fine-structure-based models for improving hearing implants, 230 k€.
- Dusdasz and A. Schrott-Fischer, FWF, Neurotrophins in inner ear and head and neck cancer, 250 k€.
Collaborations
Helge Rask-Andersen, Uppsala University, Uppsala, Sweden
Pascal Senn, Hôpitaux Universitaires de Genève, Geneva, Switzerland
Werner Hemmert, Technical University of Munich, Munich, Germany
Christoph Arnoldner, Medical University of Vienna, Vienna, Austria
Hubert Löwenheim, University of Tübingen, Tübingen, Germany
Shin-ichi Usami, Shinshu University School of Medicine, Matsumoto, Japan
Giovanni Blandino, IRCSS Regina Elena National Cancer Institute, Rome, Italy
Andrea Ladanyi, National Institute of Oncology, Budapest, Hungary