
Anichstraße 35
6020 Innsbruck
Fax: +43 512-504-24371
Email: andreas.kolk@i-med.ac.at
Website: http://www.zmk-innsbruck.at/
Research Branch (ÖSTAT Classification)
Keywords
Biological Surfaces, cell cycle inhibitors, Cranio-Maxillofacial and Oral Surgery, craniofacial implants, Nano Technology, Oncolytic Virotherapy, Osseointegration, Reconstructive Surgery, Tissue Engineering, TMJ Surgery, and Trauma Surgery
Research Focus
Reversing impaired healing of irradiated bone by the use of immobilised growth factors on nanostructured osteosynthetic material. Smart Implants- Monitoring of osseous healing and bone remodelling in vivo. VascuBone- Development of a toolbox for tailored angio-inductive or vascularised Bone Implants. Development of YB-1 based oncolytic virus for the combination- treatment of HNSCC.
General Facts
The MKG Molecular Research Unit investigates a combinational therapy using oncolytic viruses and different cancer therapeutics. For this, cell culture and molecular analyses are conducted to prove the effectivity of the therapy and evaluate the molecular background.
The consequences of radiation therapy in head and neck tumour patients is a major focus of the Department of Cranio-Maxillofacial and Oral Surgery as all aspects of wound healing including cellular behaviour, blood flow and stem cell activation are influenced by radiation.
Using smart implants as a new sensing technology allows us to gain an insight into bone healing based on serial analysis of impedance spectroscopic examinations before radiographic or histologic changes are detectable.
Reconstructive Medicine:
Reconstructive facial surgery is one focus of the Department of Cranio-Maxillofacial and Oral Surgery. After ablative tumour surgery or resection of osteonecrotic and infected bone, free tissue transplants are microvascularly anastomosed for facial rehabilitation. Research focuses on the development of minimally invasive or artificial transplants.
To achieve the goal, the Department is part of the FP-7 framework project “Vascubone” together with a further 14 partners. The department is a leader in the field of preclinical trials, provides the technology for hard tissue histology and immohistochemistry and plans and conducts animal trials.
Research
1. Innovative treatment concepts in head and neck tumours
Prof. Holm, Shasta Pelzer, Felicia Segeth
The entry of oncolytic viruses in tumour therapy is opening ground-breaking changes in current and future treatment regimens. Despite this powerful mode of action against cancer, clinical studies have shown reduced replication of OVs in target cancer cells and subsequently limited efficacy as monotherapy. However, the good combinability of OVs with other interventions has the capacity to counteract this drawback without increasing toxicity. Thus, the development of effective combinatorial treatment strategies is expected to be one of the keys to success, in establishing OVs in a future clinical routine treatment regimen. In addition, it was discovered that the overall anti-tumour activity of oncolytic viruses not only relies on their direct killing effects on local cancer cells, but also on initiating an impressive systemic immune response (Figure 1).
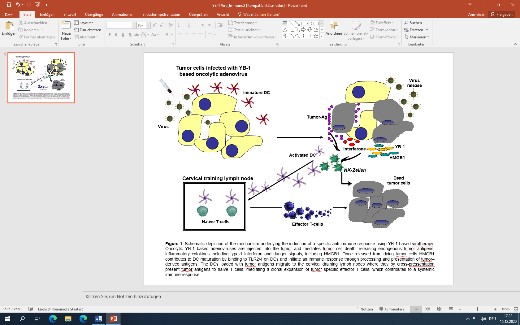
Thus, the capacity of the oncolytic virus to kill cancer cells and activating an anti-tumour immunity in the process make a combination approach with existing immune therapy e.g. immune checkpoint inhibitors and cell cycle inhibitors, very promising. Also the tumour vascularisation is addressed by specific imaging modality. (Figure 2)
Taking this into account, we have established a new, rapidly growing research unit in our Department since summer 2020. The head of the new MKG-Research-Unit is Univ.-Prof. Dr. Per Sonne Holm, an international recognised expert in the field of oncolytic virotherapy.
2. Bioactive Surfaces in Cranio-Maxillofacial and Oral Surgery
Implants have revolutionised patient care in all fields of medicine and dentistry. Titanium has evolved as the leading raw material when the treatment of bone and cartilage diseases / degeneration as well as tooth loss necessitates osseointegration of individualised tissue replacement options.
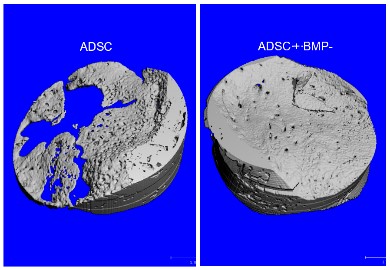
This is mainly due to its bio-inert behaviour and characteristics (tautology –use either behaviour or characteristics, but not both)-or could use properties). Titanium implants in particular provide excellent results in healthy young and adult patients. However, the success rate of any implant is hampered if the implant site is affected by poor bone wound healing. Due to conditions affecting bone turnover and homeostasis, such as osteoporosis, age, radiation therapy, bisphosphonate intake, infection, severe trauma or other pathology-related bone changes, osseous healing at the implant site is frequently limited. To overcome shortcomings of bone healing and osseointegration we used bioactive BMP-2 delivered from various coatings like nano-crystalline DIAMOND (NCD) coated implants based on nanotechnology and physisorption. Furthermore, various plasmids of BMP-2, including the RNA as the copy of the DNA, have been used as an alternative bioactive coating which, in terms of safety concerns, is an approach that has already been patented and tested in humans. Stabilised Non-Immunogenic Messenger RNA The therapeutic principle is the replacement of the missing transcript. Broad applicability from inherited diseases to regenerative medicine. Compared to the recombinant growth factor itself, the immunogenic activity of the RNA is reduced by a rate >90% (Figure 3).
3. Efficacy of arthroscopy with platelet-rich plasma versus arthroscopy alone in temporomandibular joint arthritis: A randomised controlled trial.
Patients with craniomandibular dysfunction (CMD) with a predominantly arthrogenic component can benefit from a number of minimally invasive surgical approaches after previously unsuccessful conservative treatment. The aim of various ongoing studies at our department is to evaluate the spectrum of minimal invasive surgical procedures in the treatment of temporomandibular joint (TMJ) disorders. Patients are allocated to treatment arms by using a software randomisation program; receiving either an injection of 2 ml of PRP (IMP) under direct visualisation into the superior joint space or 2 ml of lactated Ringer’s solution (placebo)
All subjects are blinded to the application of the IMP (single-blind study). The endpoint is the painless time after application and the amount of pain free TMJ-mobility. The second clinical trial is currently ongoing to evaluate the impact of orthognathic surgery on the TMJ.
4. Development of a new algorithm for evaluation of ongoing changes of the TMJ morphology via KI
Condylar changes are often due to orthodontic long-term treatment that can end up in a complete condylar resorption. To forecast this event we develop an algorithm based on the deep learning procedure on 200 3 D imaging data sets.
Pictures
Selected Publications
Temporal Tendinitis in Craniomandibular Dysfunction (CMD) – Does it Really Exist? A Temporomandibular MRI Investigation. Stimmer H, Grill F, Waschulzik B, Nieberler M, Wolff KD, Kolk A. Rofo. 2022 Nov;194(11):1242-1249. doi: 10.1055/a-1829-6134
Factors Associated with Postoperative Delirium in Patients Undergoing Complex Head and Neck Flap Surgery. Kolk A, Schwarzer C, Wolff KD, Grill F, Weingart J. J Oral Maxillofac Surg. 2022 Feb;80(2):372-379.e5. doi: 10.1016/j.joms.2021.08.153. Epub 2021 Aug 25.
Temporal Tendinitis in Craniomandibular Dysfunction (CMD) – Does it Really Exist? A Temporomandibular MRI Investigation. Stimmer H, Grill F, Waschulzik B, Nieberler M, Wolff KD, Kolk A. Rofo. 2022 Nov;194(11):1242-1249. doi: 10.1055/a-1829-6134
The Oncolytic Adenovirus XVir-N-31, in Combination with the Blockade of the PD-1/PD-L1 Axis, Conveys Abscopal Effects in a Humanized Glioblastoma Mouse Model.. Moritz Klawitter , Ali El-Ayoubi , Jasmin Buch, Jakob Rüttinger, Maximilian Ehrenfeld , Eva Lichtenegger, Marcel A Krüger, Klaus Mantwill, Florestan J Koll, Markus C Kowarik , Per Sonne Holm, Ulrike Naumann. Int J Mol Sci. 2022;23:9965. doi: 10.3390/ijms23179965
Targeting the Retinoblastoma/E2F repressive complex by CDK4/6 inhibitors amplifies oncolytic potency of an oncolytic adenovirus. Koch J, Schober SJ, Hindupur SV, Schöning C, Klein FG, Mantwill K, Ehrenfeld M, Schillinger U, Hohnecker T, Qi P, Steiger K, Aichler M, Gschwend JE, Nawroth R, Holm PS. Nat Commun. 2022:4689. doi: 10.1038/s41467-022-32087-5.
Concepts in Oncolytic Adenovirus Therapy. Mantwill K, Klein FG, Wang D, Hindupur SV, Ehrenfeld M, Holm PS, Nawroth R.Int J Mol Sci. 2021;22:10522. doi: 10.3390/ijms221910522.
Targeting Cell Cycle Facilitates E1A-Independent Adenoviral Replication. Ehrenfeld M, Segeth F, Mantwill K, Brockhaus C, Zhao Y, Ploner C, Kolk A, Gschwend JE, Nawroth R, Holm PS. J Virol. 2023: e0037023. doi: 10.1128/jvi.00370-23
The Oncolytic Adenovirus XVir-N-31 Joins Forces with CDK4/6 Inhibition Augmenting Innate and Adaptive Antitumor Immunity in Ewing Sarcoma. Schober SJ, Schoening C, Eck J, Middendorf C, Lutsch J, Knoch P, von Ofen AJ, Gassmann H, Thiede M, Hauer J, Kolk A, Mantwill K, Gschwend JE, Burdach SEG, Nawroth R, Thiel U, Holm PS. Clin Cancer Res. 2023;29(10):1996-2011. doi: 10.1158/1078-0432.CCR-22-1961..
Selection of Funding
Collaborations
Max Planck Institute for Colloids and Interfaces, TU Berlin, Berlin, Germany
Department of Health and Environment, Nanosystems, Austrian Institute of Technology, Vienna, Austria
Department of Biomedical Aging Research, University of Innsbruck, Innsbruck, Austria
Institute of Clinical Neurobiology, University Hospital Wuerzburg, Germany
Laboratory of Molecular Neuro-Oncology, Department of General Neurology, Hertie Institute for Clinical Brain Research, University of Tübingen, Germany
Department of Proteomics and Signal Transduction, Max Planck Institute of Biochemistry, 82152 Martinsried, Germany
Department of Urology, Klinikum rechts der Isar, Technical University Munich, Munich, Germany