Müllerstraße 59
6020 Innsbruck
Fax: +43 512-9003 73170
Email: lars.klimaschewski@i-med.ac.at
Website: https://www.i-med.ac.at/neuroanatomy/
Research Branch (ÖSTAT Classification)
301405, 301402, 301403, 301407
Keywords
Degeneration, Endosomal Transport, Fibroblast Growth Factors, Plasticity, Proliferation, Receptor Tyrosine Kinases, Regeneration, Signalling, Sprouty, and Survival
Research Focus
Our laboratory investigates the role of growth factors in the nervous system under normal and pathological conditions. We aim to modify signalling pathways downstream of receptor tyrosine kinases in neurons, glia and glioblastoma that may act as targets to activate pro-regenerative mechanisms in the diseased nervous system or to inhibit tumour growth of glioblastoma. Our research focuses on signalling and transport mechanisms of fibroblast growth factor receptor 1 and its inhibitor Sprouty2.
General Facts
The Institute of Neuroanatomy offers lectures and seminars in functional as well as comparative neuroanatomy for MD and PhD students. We primarily investigate the morphological consequences of growth factor signalling and receptor tyrosine kinase transport in the nervous system. Using biochemical, molecular and cellular methods in combination with high-resolution imaging, we study fundamental neurobiological phenomena such as axon outgrowth, nerve regeneration and glial/glioblastoma proliferation in collaboration with local and international research groups.
Research
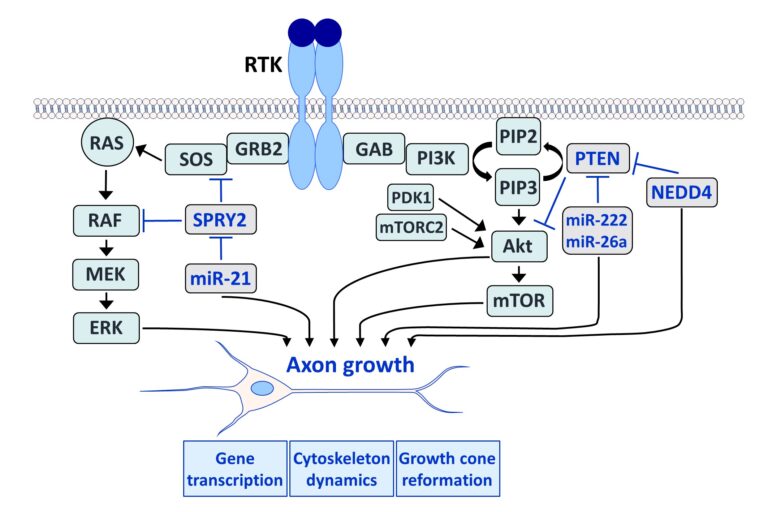
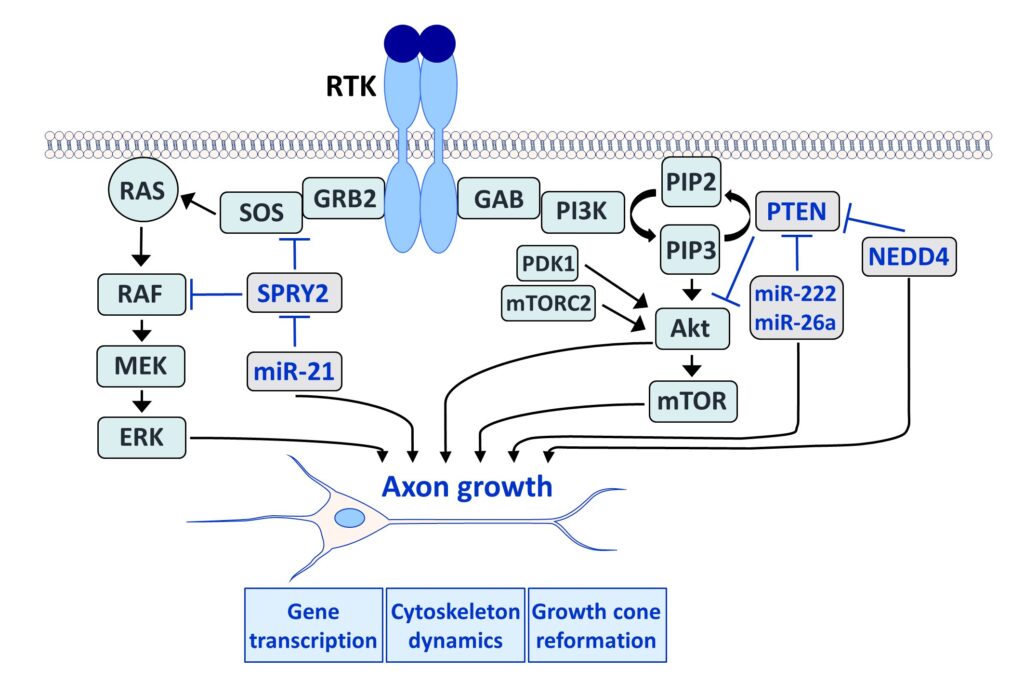
Fibroblast growth factors (FGFs) are important for the development and repair of the nervous system. Stimulation of FGF receptors (FGFRs) promotes neurogenesis, neuronal protection, axonal regeneration and remyelination in the injured nervous system. Our research focuses on the signalling and transport of FGFR1, the predominant FGFR in the nervous system. Signalling and trafficking of this receptor tyrosine kinase (RTK) have a significant influence on neuronal survival and axon outgrowth (Klimaschewski and Claus, 2021).
Among the negative feedback inhibitors of FGFR1-induced signalling are the Sprouty (SPRY proteins), which comprise a family of four homologous molecules. Knockdown of SPRY2 and 4 promotes recovery from peripheral nerve or brain lesions. Applying different in vivo models, we have demonstrated that the reduction of Sprouties in neurons or glial cells improves neuronal survival and axonal regeneration in the central and peripheral nervous system (Hausott et al., 2022).
We have demonstrated that SPRY2 knockdown enhances axon outgrowth of sensory neuron cultures and elevates extracellular signal-regulated kinase (ERK) activity. Heterozygous SPRY2+/- knockout mice recover faster in response to a sciatic nerve crush, and homozygous SPRY2-/- mice reveal enhanced mechanosensory function (Hausott et al., 2009; Marvaldi et al., 2015). Dual-interference with SPRY2 and phosphatase and tensin homolog deleted on chromosome 10 (PTEN), an inhibitor of the phosphoinositide 3-kinase (PI3K)/Akt pathway, promotes axon elongation stronger than the knockdown of each molecule individually (Jamsuwan et al. 2020). In the brain, siRNAs against Sprouties reduce the lesion size in response to endothelin-induced vasoconstriction, a model for stroke (Klimaschewski et al., 2016). Secondary brain damage in response to kainate-induced excitotoxicity in the hippocampus is also significantly diminished in mice with reduced SPRY 2/4 levels (Thongrong et al., 2016).
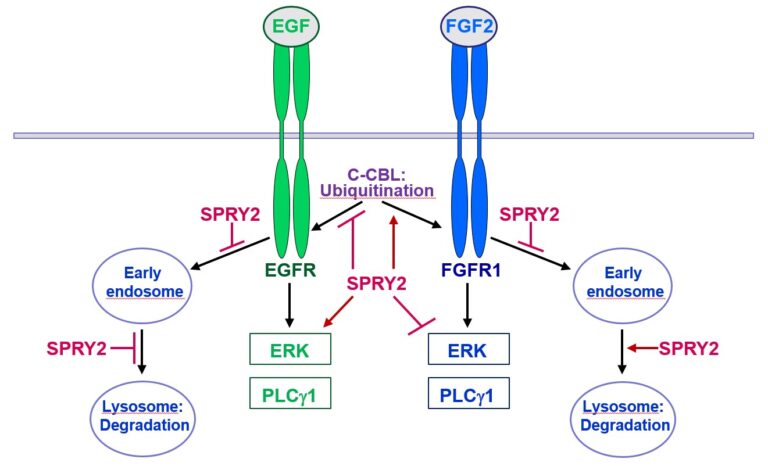
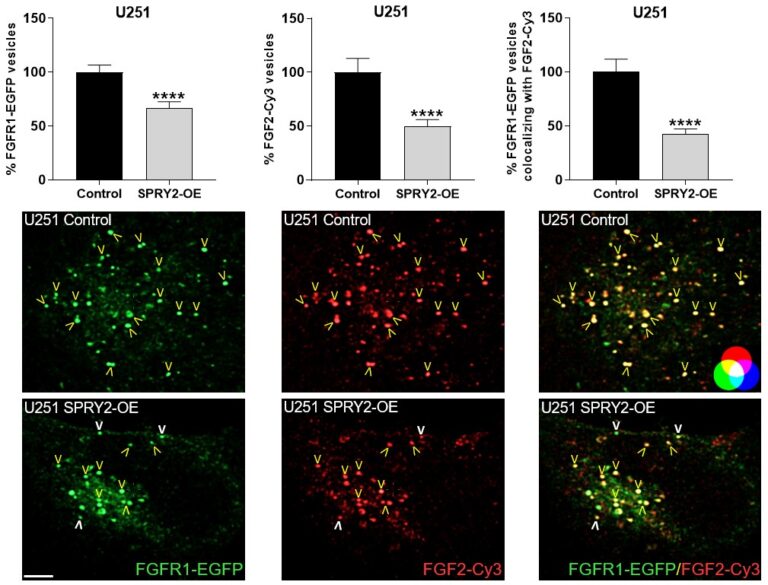
Furthermore, SPRY2 is a key regulator of tumour formation in the brain. It is up-regulated in malignant glioblastoma (GBM) and this correlates with the reduced survival of patients. Knockdown of SPRY2 inhibits GBM tumour growth via increased ERK and Akt activation, which leads to premature S-phase entry and increased DNA damage (Park et al., 2018). SPRY2 inhibits endocytosis of FGFR1 in GBM cells and reduces FGFR1 protein through increased c-casitas b-lineage lymphoma (c-CBL)-mediated ubiquitination, which enhances lysosomal degradation of the receptor and reduces FGF2-induced activation of ERK and phospholipase Cg1 (PLCg1). By contrast, SPRY2 increases epidermal growth factor receptor (EGFR) levels in GBM cells and stimulates EGF-induced activation of ERK and PLCg1 (Hausott et al., submitted). Together these data reveal a prominent role of SPRY2 in GBM.
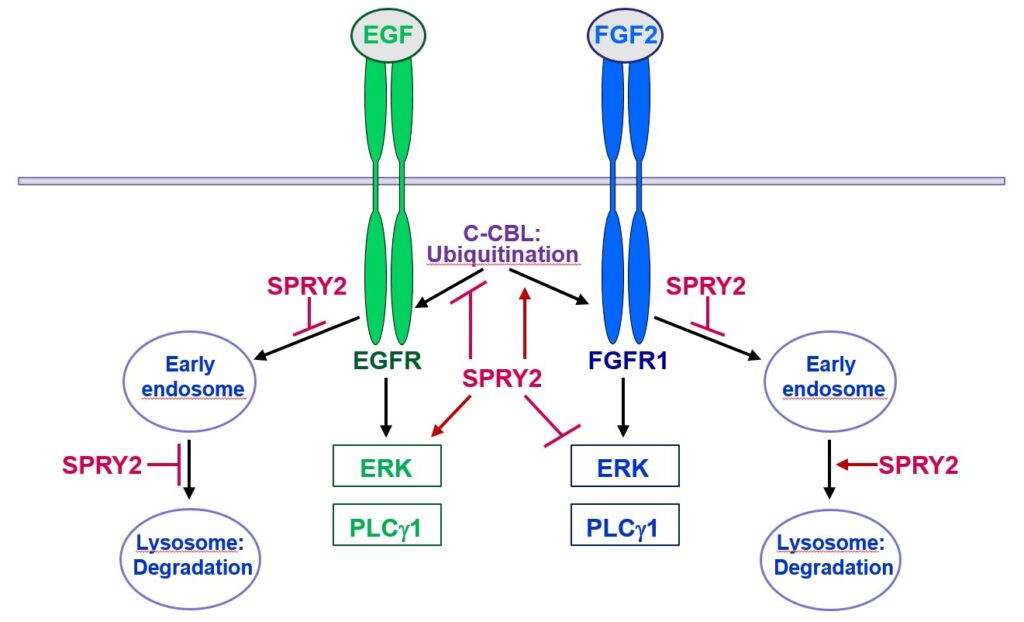
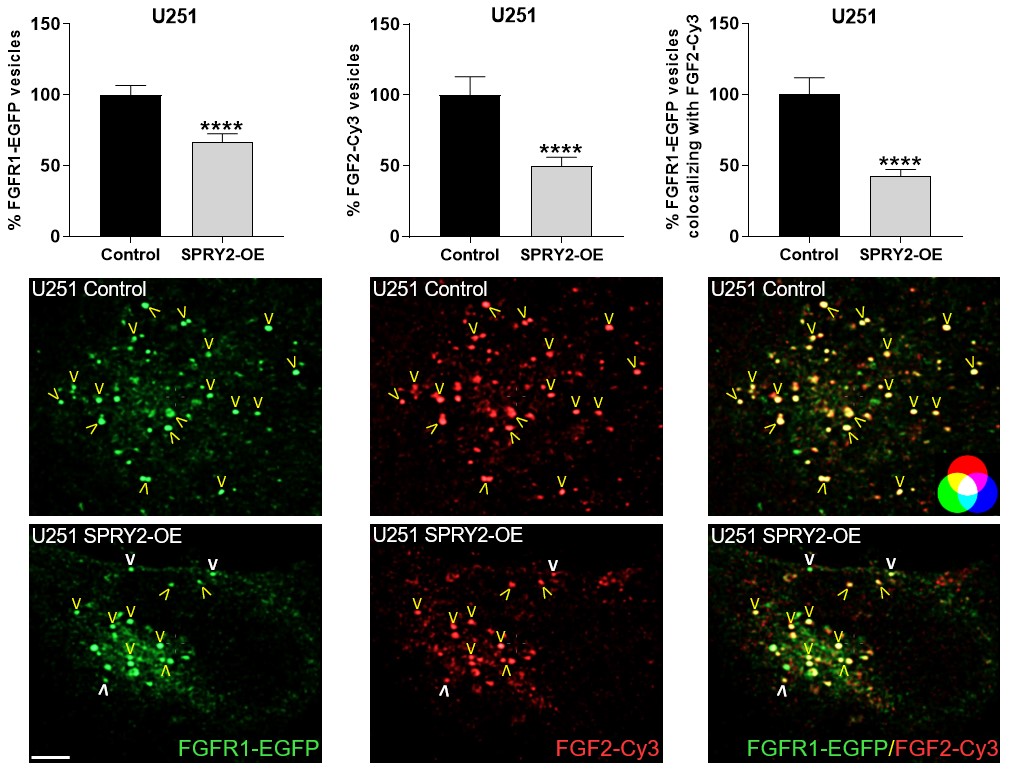
Together, Sprouties may provide a novel therapeutic target to improve regeneration in the lesioned nervous system and to inhibit malignant tumour growth in the brain.
Pictures
Selected Publications
Signal Transduction Regulators in Axonal Regeneration. Hausott B, Glueckert R, Schrott-Fischer A, Klimaschewski L. Cells. 2022 May 4;11(9):1537. doi: 10.3390/cells11091537.
Innsbruck’s histological institute in the third Reich: Specimens from NS-victims. Freilinger M, Klimaschewski L, Brenner E. Ann Anat. 2022 Apr;241:151890. doi: 10.1016/j.aanat.2022.151890.
Lentivirus- or AAV-mediated gene therapy interventions in ischemic stroke: A systematic review of preclinical in vivo studies. Skukan L, Brezak M, Ister R, Klimaschewski L, Vojta A, Zoldoš V, Gajović S. J Cereb Blood Flow Metab. 2022 Feb;42(2):219-236. doi: 10.1177/0271678X211039997. Epub 2021 Aug 24.
Parkinson’s and Alzheimer’s Today: About Neurodegeneration and its Therapy. Klimaschewski L. Springer. 2022 Dec. Book. ISBN-10: 3662663686, ISBN-13: 978-3662663684
Fibroblast Growth Factor Signaling in the Diseased Nervous System. Klimaschewski L, Claus P. Mol Neurobiol. 2021 Aug;58(8):3884-3902. doi: 10.1007/s12035-021-02367-0.
Selection of Funding
FWF, P 28909: Sprouty2 and receptor tyrosine kinase trafficking (Barbara Hausott)
Collaborations
P. Claus, SMATHERIA gGmbH – Non-Profit Biomedical Research Institute, Hannover, Germany
E. Haugsten, Department of Biochemistry, Institute for Cancer Research, Norway
S. Gajovic, Laboratory for Regenerative Neuroscience, University of Zagreb, Croatia
P. Krejčí, Department of Biology, Masaryk University Brno, Czech Republic