Peter Mayr Straße 1A
6020 Innsbruck
Fax: +43 (0)512 9003 73518
Email: Gottfried.Baier@i-med.ac.at
Website: https://www.i-med.ac.at/cell-genetics/
Research year
Research Branch (ÖSTAT Classification)
301301, 301902, 302055
Keywords
cancer immunity, Immune cell signalling, innovative immunotherapy approaches, and T cell effector/memory functions
Research Focus
Our aim is to understand the selective functions of defined signalling pathways in immune cells, in particular CD4+ and CD8+ T lymphocytes, and to use the information to develop strategies for manipulating the immune response, either to promote immunosuppression in the context of autoimmune diseases and transplant rejection, or to stimulate immunity as part of innovative cancer immunotherapy approaches.
Mechanistic understanding of T-cell effector function in host protective tumour immunity
We need to decipher the biochemical processes that integrate signals from antigens, cytokines, integrins and inhibitory receptors to understand the physiology and pathophysiology of T lymphocytes. The cell genetics team has expertise in mouse genetics, effector/memory T-cell differentiation and molecular signalling processes and is undertaking hypothesis-driven mechanistic studies as well as unbiased CRISPR/Cas9-based genetic screens and scRNA sequencing.
The cell genetics team was the first to identify the T lymphocyte intrinsic PKCtheta/NR2F6/Cbl-b axis as an essential signalling node that governs the complex host-tumour interactions at the inflammation-cancer interface. Modulation of this pathway renders effector T cells able to reject otherwise lethal tumour loads and their metastases in preclinical cancer model systems. One of our goals is to elucidate the inter- and intracellular mechanisms that remodel the immune context to enable superior tumour rejection.
General Facts
Our research is focused on late-stage lung cancer and melanoma, with the aim of enhancing the anti-tumour immune response.
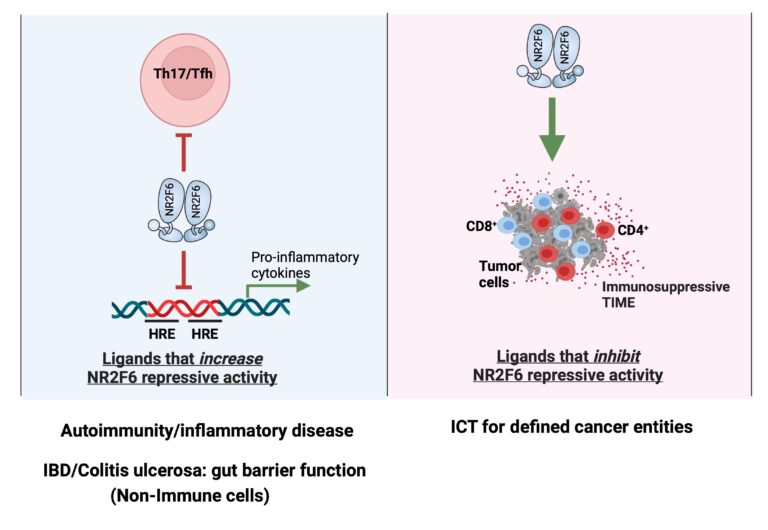
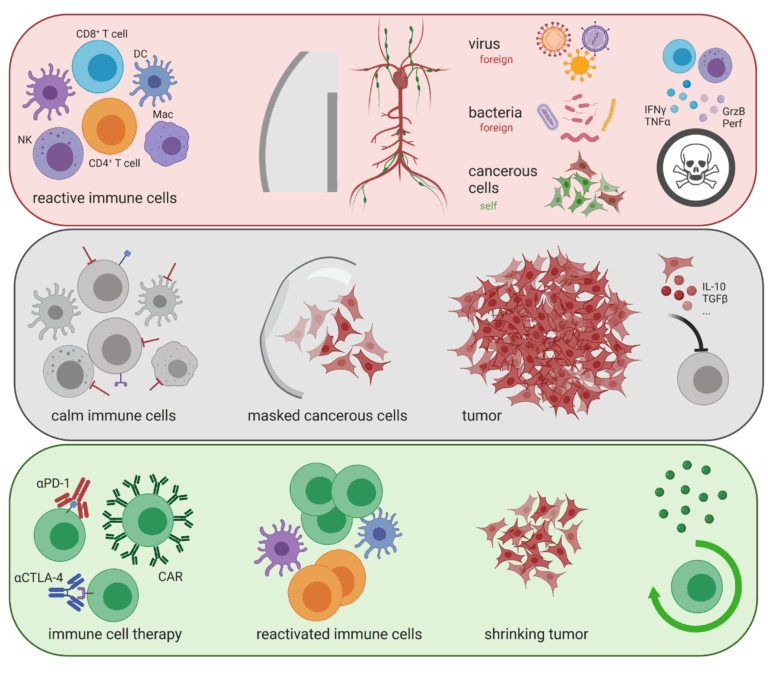
Targeting T-cell surface receptors such as CTLA-4 and/or PD-1 with recombinant antibodies has been the most successful strategy for cancer immunotherapy. However, a significant proportion of patients fail to respond to the regimens. Using germline gene ablation and CRISPR/Cas9-mediated acute gene mutagenesis, we have characterized the orphan nuclear receptor NR2F6 (nuclear receptor subfamily 2 group F member 6) as an intracellular immune checkpoint in the effector T-cell compartment and have shown that it can improve anti-tumour immunotherapy responses, particularly in combination with CTLA-4 and PD-1 inhibition. Preclinical experimental evidence defines key protein-protein and protein-DNA interactions that strongly support the immune function of lymphatic NR2F6 in tumour immune evasion. Targeting NR2F6 appears particularly suited to improving the efficacy and broadening the applicability of cancer immunotherapy and thus has the potential to strengthen the immune system of tumour patients in future clinical trials.
Research
AG Gruber: Novel role of Nemo-like kinase in cancer immunity
A major obstacle to the therapeutic success of T cell-based cancer immune therapies is a phenomenon known as “T cell exhaustion”, a state of reduced effector function. We have compelling new experimental evidence that Nemo-like kinase (NLK) has a role in an ‘exhaustion avoidance pathway’ in T cells. Based on these results, we are working on NLK-mediated signalling in T cells, with a special focus on T-cell exhaustion during antitumour immunity.
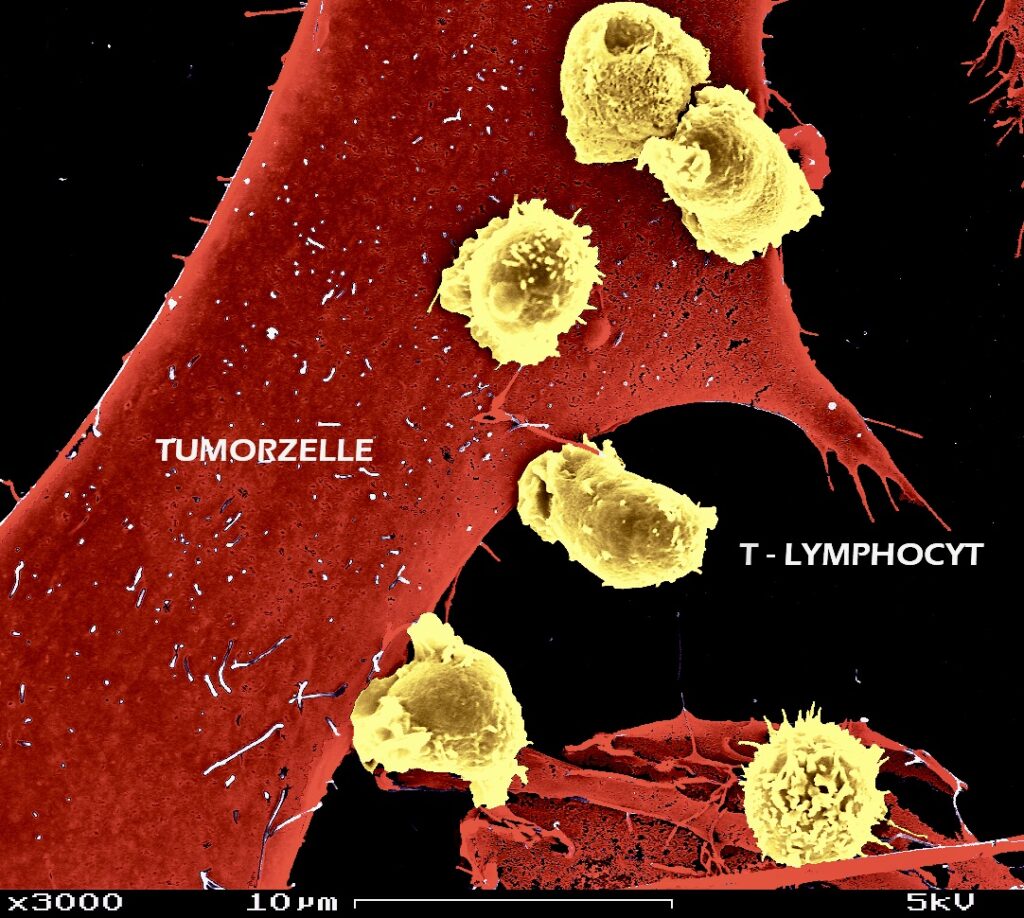
AG Klepsch: Identification of new pathways in cancer immunity
The future of the development of immuno-oncology therapies lies in combination regimens. Using both rational approaches and CRISPR/Cas9 pooled library screens in mice, we are exploring novel target candidates with a function in the physiology and pathophysiology of T lymphocytes.
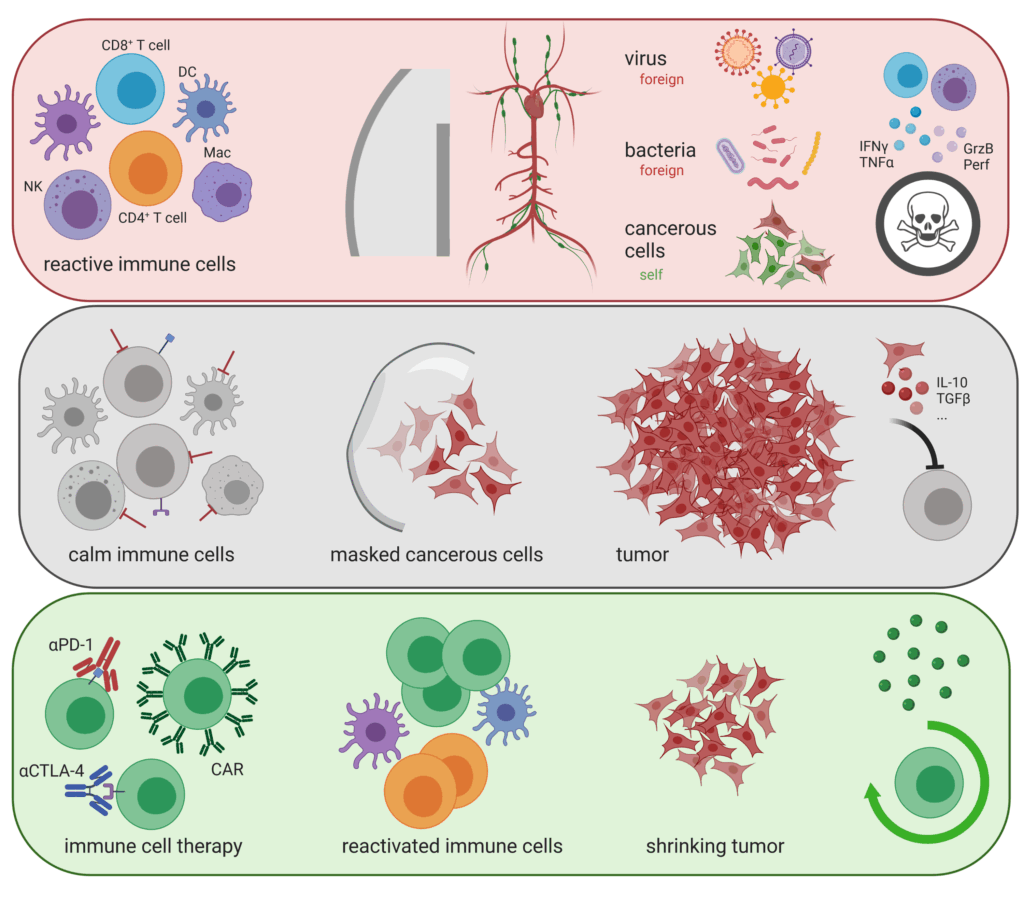
AG Bellaire-Siegmund: Role of selected protein kinases in adaptive immunity
Our goal is to understand the selective functions of defined signal transduction pathways in CD4+ and CD8+ T lymphocytes mediated by protein kinases such as PKC and PKD.
AG Thuille: Role of metabolic regulation in T-cell biology
Our aim is to decipher the biochemical processes of metabolic regulation that integrate signals received from antigen, cytokine and integrin receptors as well as inhibitory receptors in primary T cells.
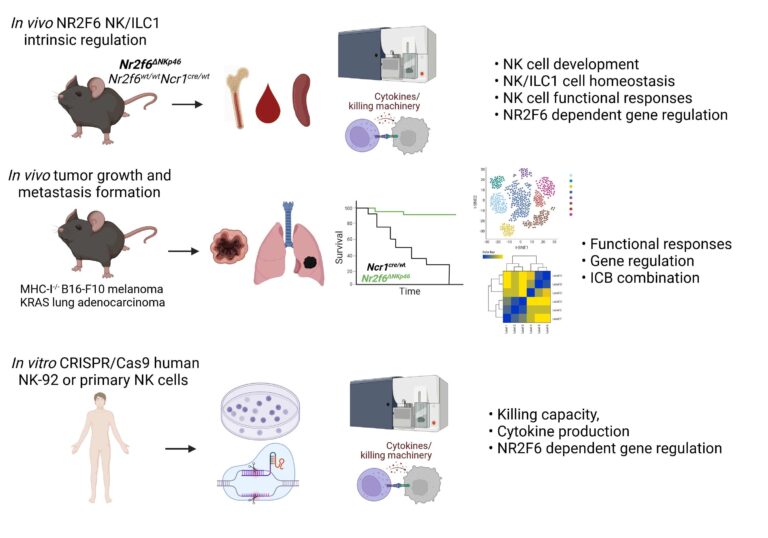
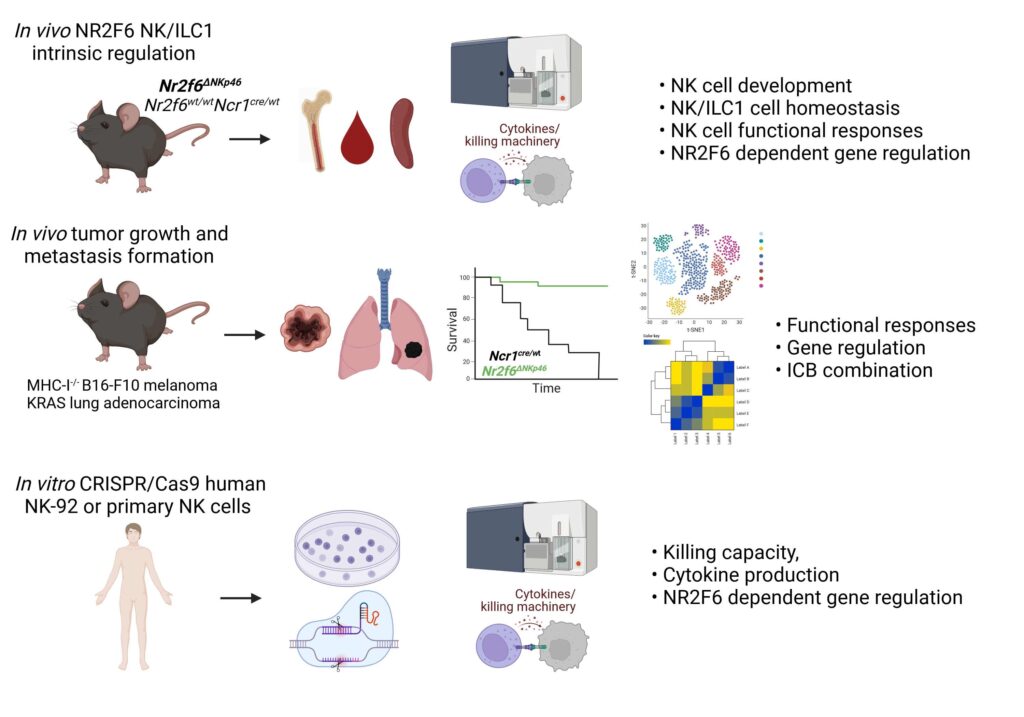
AG Kleiter: Novel role of NR2F6 in NK-cell antitumor responses
Nr2f6-deficient mice are protected against MHC-I-negative B16-F10 melanoma lung metastases, particularly by IL-15 complex treatment, suggesting a role for NK-intrinsic NR2F6 function. We will determine whether and how NR2F6 influences NK cell-mediated tumour surveillance in mice and humans, aiming to identify the pathway(s) responsible.
Pictures
Selected Publications
1: Woelk J, Hornsteiner F, Aschauer-Wallner S, Stoitzner P, Baier G, Hermann-
Kleiter N. Regulation of NK cell development, maturation, and antitumour
responses by the nuclear receptor NR2F6. Cell Death Dis. 2025 Feb 7;16(1):77.
doi: 10.1038/s41419-025-07407-4. PMID: 39920136; PMCID: PMC11806049.
2: Zur Nedden S, Safari MS, Weber D, Kuenkel L, Garmsiri C, Lang L, Orset C,
Freret T, Haelewyn B, Hotze M, Kwiatkowski M, Sarg B, Faserl K, Savic D,
Skvortsova II, Krogsdam A, Carollo S, Trajanoski Z, Oberacher H, Zlotek D,
Ostermaier F, Cameron A, Baier G, Baier-Bitterlich G. Protein kinase N1
deficiency results in upregulation of cerebral energy metabolism and is highly
protective in in vivo and in vitro stroke models. Metabolism. 2024
Dec;161:156039. doi: 10.1016/j.metabol.2024.156039. Epub 2024 Sep 26. PMID:
39332493.
3: Bosch M, Kallin N, Donakonda S, Zhang JD, Wintersteller H, Hegenbarth S, Heim
K, Ramirez C, Fürst A, Lattouf EI, Feuerherd M, Chattopadhyay S, Kumpesa N,
Griesser V, Hoflack JC, Siebourg-Polster J, Mogler C, Swadling L, Pallett LJ,
Meiser P, Manske K, de Almeida GP, Kosinska AD, Sandu I, Schneider A,
Steinbacher V, Teng Y, Schnabel J, Theis F, Gehring AJ, Boonstra A, Janssen HLA,
Vandenbosch M, Cuypers E, Öllinger R, Engleitner T, Rad R, Steiger K, Oxenius A,
Lo WL, Klepsch V, Baier G, Holzmann B, Maini MK, Heeren R, Murray PJ, Thimme R,
Herrmann C, Protzer U, Böttcher JP, Zehn D, Wohlleber D, Lauer GM, Hofmann M,
Luangsay S, Knolle PA. A liver immune rheostat regulates CD8 T cell immunity in
chronic HBV infection. Nature. 2024 Jul;631(8022):867-875. doi:
10.1038/s41586-024-07630-7. Epub 2024 Jul 10. PMID: 38987588; PMCID:
PMC11269190.
4: Koutník J, Peer S, Humer D, Sumara G, Leitges M, Baier G, Siegmund K. T cell-
intrinsic PKD3 fine-tunes differentiation into CD8<sup>+</sup> central memory T
cells and CD8 single positive thymocyte development. Immunology. 2024
Sep;173(1):125-140. doi: 10.1111/imm.13804. Epub 2024 May 26. PMID: 38798068.
5: Kim H, Feng Y, Murad R, Pozniak J, Pelz C, Chen Y, Dalal B, Sears R,
Sergienko E, Jackson M, Ruppin E, Herlyn M, Harris C, Marine JC, Klepsch V,
Baier G, Ronai ZA. Melanoma-intrinsic NR2F6 activity regulates antitumour
immunity. Sci Adv. 2023 Jul 7;9(27):eadf6621. doi: 10.1126/sciadv.adf6621. Epub
2023 Jul 5. PMID: 37406115; PMCID: PMC10321753.
Selection of Funding
- Austrian Science Funds (FWF) P34368 Role of protein kinase D in T cell biology, (Kerstin Bellaire-Siegmund), 2021-2025
- Austrian Science Funds (FWF) T-1292 Firnberg-Programm Darmbakterien manipulieren Immunantwort gegen Krebs, (Victoria Klepsch), 2021-2024
- ERC Proof of concept grant, CAR-T-uning: Tuning Chimeric Antigen T Cell (CAR-T) therapy to lung cancer Acronym: CAR-T(uning) (Coord. Gottfried Baier), 2022-2024
- ÖAW’s DOC research scholarship, Potentiating CD8 CAR-T cells by targeting of the lymphatic PKD3 pathway, (Jiri Koutnik), 2022-2024
- FFG Bridge grant #49465145, Extension of CAR-T cell therapy success by CBLB pathway blockade (EXCEL-T; Coord. Gottfried Baier), 2023-2025
- ERC Proof of concept grant #101189004, NR2F6-AIM – NR2F6 Blockade as Adoptive Immune Cell Therapy for Metastatic Melanoma (Gottfried Baier), 2024-2025
- Austrian Science Funds #FWF PAT 9292223: The role of the Nemo-like kinase signalling pathway in the avoidance of T cell exhaustion during antitumour immunity (Thomas Gruber), 2024-2028
- Austrian Science Funds #FWF TAI 7132824: Umkehrung der altersbedingten Immunoseneszenz (Coord. Gottfried Baier), 2024-2026
- Austrian Science Funds #FWF PAT 601462: NK-intrinsic role of NR2F6 in tumour immunity (Natascha Kleiter), 2024-2028 Austrian Science Funds #FWF PAT 1317523: A2AR pathway as a candidate pathological driver of experimental cerebral malaria (Coord. Gottfried Baier), 2024-2028
Collaborations
Ze’ev Ronai, Sanford Burnham Medical Research Institute, San Diego, USA
Amnon Altman, La Jolla Institute for Immunology, San Diego, USA
Michael Leitges, Memorial University of Newfoundland, St. John`s, Canada
Josef Penninger, Life Sciences Institute, Vancouver Campus, Univ. of British Columbia, Canada
Wallace Langdon, University of Western Australia, Perth, AUS
Jürgen Wagner; Novartis Pharma, Basel, Switzerland
Noah Isakov, Ben Gurion University of the Negev, Israel
Shoji Yamamoto, Daiichi Sankyo Ltd., Tokyo, Japan