Anichstraße 35
6020 Innsbruck
Fax: +43 51250424199
Email: michael.joannidis@i-med.ac.at
Website: https://www.i-med.ac.at/notfallmedizin/
Research Branch (ÖSTAT Classification)
3519, 301205, 301203
Keywords
Acute kidney injury, antimicrobial agents, biomarkers, cardio pulmonary resuscitation, coagulation factors, COVID-19, pharmacodynamics, plasma pharmacokinetics, sepsis, and target-site pharmacokinetics
Research Focus
Applied clinical as well bench-to-bedside research, covering several aspects of critical illness, with special emphasis on acute kidney injury (AKI), sepsis, COVID-19 disease and cardiopulmonary resuscitation (CPR).
Definition and clinical validation of biomarkers for diagnosis and prognosis of AKI and CPR.
Identification and characterisation of interaction of endothelial with the coagulation system in systemic inflammation.
Intensive- care- specific pharmacodynamics and pharmacokinetics.
General Facts
The Joint Institution / Division of Medical Intensive Care and Emergency Medicine was established in December 2012. Clinically, it was designed as a core facility for the Department of Internal Medicine, to provide a high level of intensive care and emergency medicine. It comprises a level-three intensive care unit and medical emergency room, including a short-stay (maximum of 24 hours) ward in Medizinzentrum Anichstrasse (MZA). Administratively, the unit is affiliated to the Department of Internal Medicine I (Director: Prof. Dr. Herbert Tilg).
The unit is involved in several multicentre clinical trials to investigate early diagnosis and treatment of acute kidney injury, treatment of severe infections and sepsis as well as antimicrobial pharmacokinetics. Complementary in vitro models are used to investigate inflammatory mechanisms of renal injury.
The research unit comprises the Inflammation Research Laboratory (U-1-015), which hosts the two research groups: the Intensive Care Medicine group (Mag. Anna Brandtner, Dr. Julia Hasslacher, Dr. Sebastian Klein, PhD, Dr. Georg Lehner, PhD, Dr. Paul Köglberger, Dr. Timo Mayerhöfer, Dr. Fabian Perschinka, Dr. Birgit Zassler, Mag. Viktoria Haller, Dr. Andrea Köhler) led by Michael Joannidis and the Clinical Pharmacokinetics group (Tiziana Gasperetti, MSc., Jana Marx, MSc. und René Welte, PhD) led by Ao Univ. Prof. Dr. Romuald Bellmann.
Collaboration partners include all university clinics (I – V) in the Department of Internal Medicine, the Neurological Intensive Care Unit and the Surgical/Trauma Intensive Care Unit as well as the Hygiene and Medical Microbiology division and the Molecular and Cellular Pharmacology division.
Research
Acute Kidney Injury (AKI) (PI: Michael Joannidis, Timo Mayerhöfer, Fabian Perschinka, Paul Köglberger)
AKI occurs in > 50% of critically ill patients and is associated with significant mortality and long-term morbidity, including end-stage renal disease. Early diagnosis and prediction of recovery are expected to have a significant impact on treatment and outcome for this syndrome. We have therefore participated in several multicentre trials, which lead to the establishment of new biomarkers for stress and early diagnosis (urinary cell-cycle arrest proteins TIMP-2 and IGFBP-7) and to predict persistent AKI (chemokine ligand 14, CCL-14). This resulted in a proposal to revise current KDIGO criteria for the diagnosis of AKI by additionally including such biomarkers (1). About 5 – 10% of patients who suffer from AKI require renal replacement therapy (RRT). To define optimal timing of the initiation of RRT, we participated in the design and national coordination of the STARRT-AKI trial, which is currently the largest international multicentre randomised trial comparing accelerated versus standard initiation of RRT in critically ill patients. The study discovered increased risk for dependency on RRT at 90 days for patients in the accelerated arm, which is primarily occurring in patients with chronic kidney disease according to a secondary analysis of this study (2). Biomarkers predicting persistent versus reversible AKI (e.g. CCL-14) may help to optimise timing in an individualised approach in near future. In a complementary approach, we are currently investigating the effect of hypoxia and chronic inflammation on CCL-14 release on renal epithelial cells using an endo-epithelial co-culture system. Finally, a researcher-initiated randomised clinical trial investigating optimal composition of substitution fluids during renal replacement therapy using regional citrate anticoagulation is underway and expected to optimise treatment in patients requiring RRT (NCT04071171).
Sepsis and Septic Shock (PI: Georg Lehner)
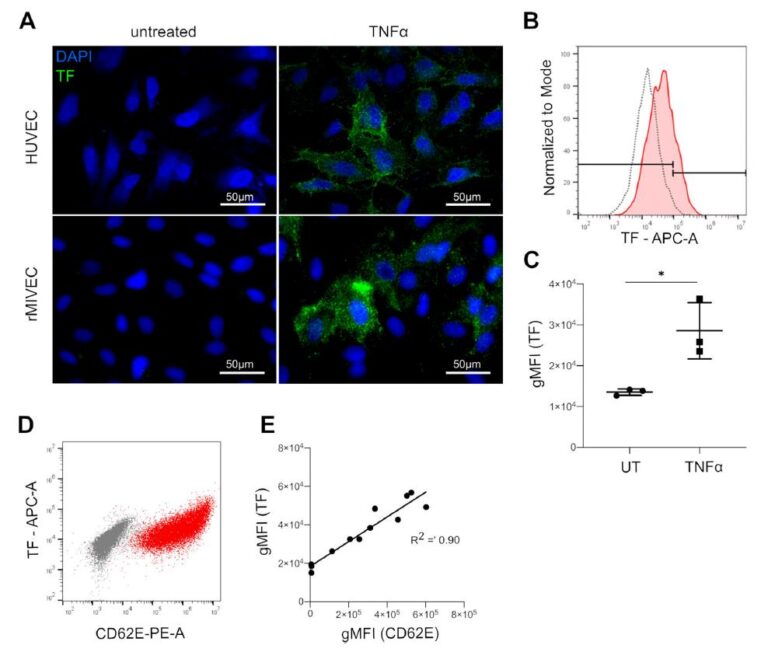
Sepsis is a syndrome that leads to organ dysfunction via multiple pathophysiological processes. The endothelium and the coagulation system are tightly linked to inflammation and play a central role in the complex syndrome. We established an in vitro coagulation test system that includes endothelial cells of different vascular beds. This test system can be used as tool to study the interaction between distinct factors and steps of the coagulation cascade and activated endothelial cells. A major initiator of the coagulation is tissue factor (TF), which can be inhibited by a tissue factor pathway inhibitor (TF). In vitro, we discovered an imbalance of TF to TFPI associated with higher TF activity specifically in activated microvascular cells
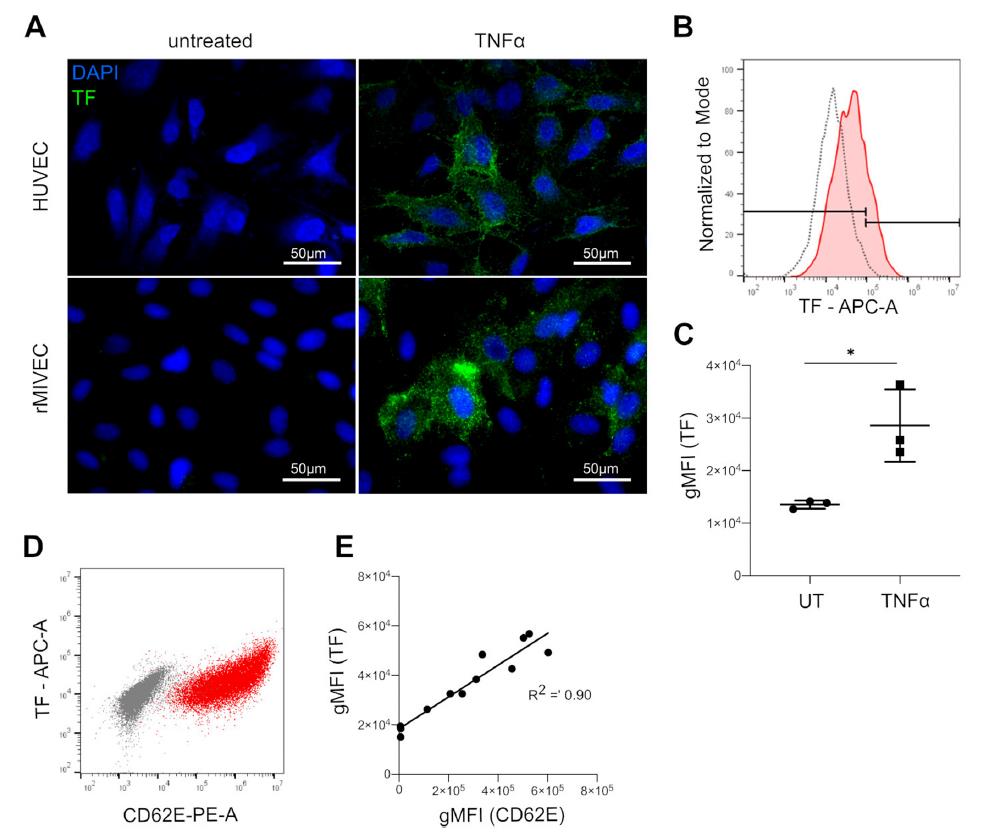
(3). This mechanism might contribute to microvascular alterations, disseminated intravascular coagulation (DIC) and organ dysfunctions in septic shock. Consequently, in a project funded by the Österreichische Gesellschaft für Internistische und Allgemeine Intensivmedizin und Notfallmedizin (ÖGIAIN), we are investigating whether DIC, organ dysfunctions and outcome are associated with alterations in the TF/TFPI ratio in patients with septic shock.
Hypoxic Brain Damage after Cardiopulmonary Resuscitation (CPR) (PI: Michael Joannidis, Julia Hasslacher)
Cardiac arrest is one of the major causes of death in cardiovascular disease and it is frequently associated with long-term neurological deficits in case of survival. In 2014, our research group identified Secretoneurin as a very sensitive and robust biomarker, which predicts unfavourable neurological outcome after CPR. As recently analysed, prediction is not influenced by the sex. Furthermore, we identified several treatment-associated factors during the post cardiac arrest syndrome such as occurrence of AKI or ventilator associated pneumonia influencing outcome of patients after successful CPR (4). Therapeutic hypothermia by actively cooling patients to a core temperature of 34° was recommend by several guidelines until recently. However, a recent large randomised controlled trial (TTM 2 trial), to which we contributed both in study design as well as by patients as the only Austrian recruiting site, strongly challenged this assumption by showing the same outcome if patients were strictly kept on normothermia (5).
COVID-19 (PI: Timo Mayerhöfer, Fabian Perschinka)
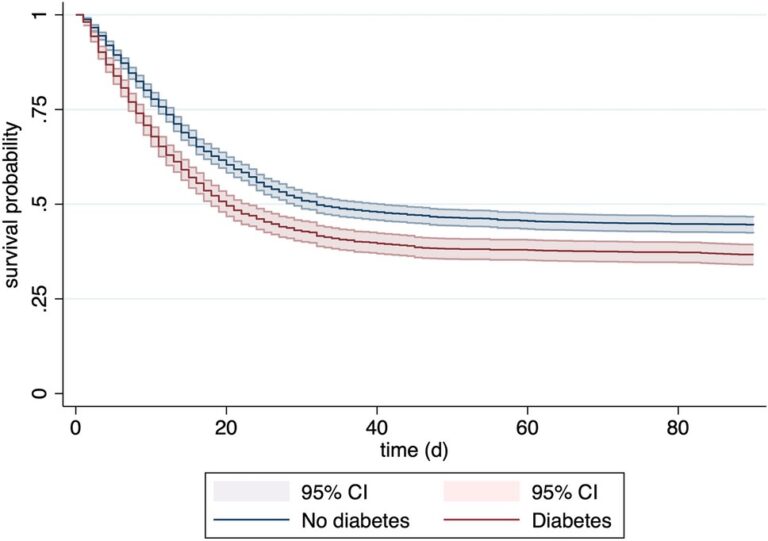
Infection with SARS-COV2 carries a significant risk of respiratory failure that often requires mechanical ventilation. The surges of COVID-19 disease placed a significant strain on intensive care bed capacities, leading to decompensation in some countries. After having established the Tyrol COVID-19 registry in 2020 we were collecting information about key factors of this disease since then. We were able to demonstrate that an ICU network structure – with a tertiary centre providing the highest level of care as a backbone – results in superior outcome and optimal resource use. We could repeatedly demonstrate that both overt and unrecognised diabetes are predominant risk factors for severe COVID-19 and impaired outcome in the cohort of Tyrol. Recently, we could reaffirm this finding also for elderly critically ill patients suffering from COVID-19 in the COVIP study, which represents a patient cohort already suffering from various comorbidities due to age
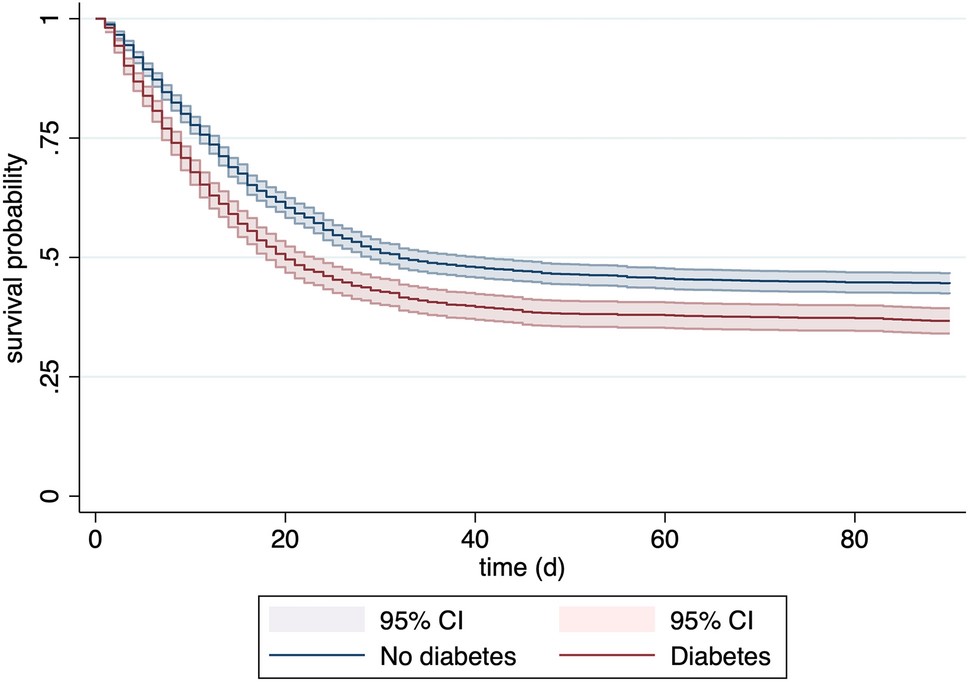
(6). Finally, when investigating COID-19-associated sepsis, we could demonstrate significant differences in phenotypes between bacterial and viral sepsis
![Fig. 2: Maximum inflammatory parameters within 48 h after ICU admission (Bacterial sepsis vs. COVID-19 [2nd wave]), IL-6 interleukin-6, PCT procalcitonin, CRP c-reactive protein.](https://researchreport.i-med.ac.at/wp-content/uploads/2023/09/Fig.-2-1024x603.jpg)
Projects of the Clinical Pharmacokinetics Unit:
Current research projects of the Clinical Pharmacokinetics Unit comprise three major topics:
Penetration of echinocandin antifungals into human tissues
Concentrations of the antifungals anidulafungin and micafungin were determined in 8 different tissues obtained during autopsy. The majority was recovered from the liver (8). Tissue concentrations of caspofungin achieved the highest levels in liver, spleen, kidney and lung. (9).
Echinocandin target-site pharmacokinetics in human body fluids
Echinocandin concentrations in wound secretion were significantly lower than the simultaneous plasma concentrations and below the MIC values of some relevant pathogens. (10). Ascites fluid concentrations were also below the simultaneous plasma levels and displayed a slower rise and decline of echinocandin concentrations in ascites fluid than in plasma (11). Accordingly, biliary concentrations were below the simultaneous plasma levels. (Reinstadler et al. in preparation). Echinocandin levels in pleural effusion were also lower than in plasma and displayed a slower rise and decline. (Welte et al. in preparation).
Target-site pharmacodynamics of echinocandins in body fluids of critically ill patients:
The antifungal activity of the echinocandins in ascites fluid was assessed by incubation of Candida (C.) albicans and C. glabrata with echinocandin-spiked native ascites or patient ascites fluid. However, no consistent eradication was achieved (11). Results for pleural effusion were similar (Welte et al. in preparation).
Pictures
Selected Publications
- Ostermann M, Zarbock A, Goldstein S, Kashani K, Macedo E, Murugan R, et al. Recommendations on Acute Kidney Injury Biomarkers From the Acute Disease Quality Initiative Consensus Conference: A Consensus Statement. JAMA Netw Open. 2020;3(10):e2019209.
- Bagshaw SM, Neto AS, Smith O, Weir M, Qiu H, Du B, et al. Impact of renal-replacement therapy strategies on outcomes for patients with chronic kidney disease: a secondary analysis of the STARRT-AKI trial. Intensive Care Med. 2022;48(12):1736-50.
- Brandtner AK, Lehner GF, Pircher A, Feistritzer C, Joannidis M. Differential procoagulatory response of microvascular, arterial and venous endothelial cells upon inflammation in vitro. Thromb Res. 2021;205:70-80.
- Hasslacher J, Steinkohl F, Ulmer H, Lehner G, Klein S, Mayerhoefer T, et al. Increased risk of ventilator-associated pneumonia in patients after cardiac arrest treated with mild therapeutic hypothermia. Acta Anaesthesiol Scand. 2022;66(6):704-12.
- Dankiewicz J, Cronberg T, Lilja G, Jakobsen JC, Levin H, Ullén S, et al. Hypothermia versus Normothermia after Out-of-Hospital Cardiac Arrest. N Engl J Med. 2021;384(24):2283-94.
- Mayerhöfer T, Klein S, Wernly B, Flaatten H, Guidet B, De Lange DW, et al. Diabetes mellitus is associated with 90-day mortality in old critically ill COVID-19 patients: a multicenter prospective observational cohort study. Infection. 2023:1-9.
- Perschinka F, Mayerhöfer T, Lehner GF, Hasslacher J, Klein SJ, Joannidis M. Immunologic response in bacterial sepsis is different from that in COVID-19 sepsis. Infection. 2022;50(4):1035-7.
- Marx J, Welte R, Gasperetti T, Moser P, Joannidis M, Bellmann R. Human Tissue Distribution of Anidulafungin and Micafungin. Antimicrob Agents Chemother. 2021;65(7):e0016921.
- Marx J, Reinstadler V, Gasperetti T, Welte R, Oberacher H, Moser P, et al. Human tissue distribution of caspofungin. Int J Antimicrob Agents. 2022;59(4):106553.
- Gasperetti T, Welte R, Oberacher H, Marx J, Lorenz I, Schellongowski P, et al. Penetration of echinocandins into wound secretion of critically ill patients. Infection. 2021;49(4):747-55.
- Welte R, Oberacher H, Gasperetti T, Pfisterer H, Griesmacher A, Santner T, et al. Pharmacokinetics and Antifungal Activity of Echinocandins in Ascites Fluid of Critically Ill Patients. Antimicrob Agents Chemother. 2021;65(7):e0256520.
Selection of Funding
- COVID-19 ICU Tyrol Registry funded by the Tiroler Landesregierung
- Tissue factor and tissue factor pathway inhibitor in sepsis- ÖGIAIN Research fund
- Target-Site Pharmacokinetics and –Activity of Echinocandins, Austrian Research funds FWF (Project KLI 565-B31), Romuald Bellmann
- The study on pharmacokinetics of trimethoprim and sulfametrole was supported Austrian Research Promotion Agency (FFG, project number 848350) and by an industrial grant.
Collaborations
- Prof. Dr. Thomas Staudinger, Intensive Care Unit, Internal Medicine I, Medical University Vienna, Vienna, Austria
- Professor Stefan Kluge, Department of Intensive Care Medicine, Hamburg Eppendorf, Hamburg, Germany
- John Kellum, MD, FCCM, FACP, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA
- Sean Bagshaw MD, PhD , Assoc. Prof., University of Alberta, Edmonton, Canada
- Peter Pickkers, Department of Intensive Care, Radboud University Medical Centre, Nijmegen, the Netherlands
- Ravindra L. Mehta, MD, UCSD Medical Centre, San Diego, CA, USA
- Nikla Nielsen, MD. Lund University, Sweden
- Philipp Eller, Department of Internal Medicine, University Hospital of Graz
- Daniel Dankl, Salzburg General Hospital and Paracelsus Private Medical University, Salzburg
- Piotr Smuszkiewicz, Department of Anesthesiology, Intensive Therapy and Pain Treatment, University Hospital Przybyszewskiego, Poznan, Poland
![Fig. 2: Maximum inflammatory parameters within 48 h after ICU admission (Bacterial sepsis vs. COVID-19 [2nd wave]), IL-6 interleukin-6, PCT procalcitonin, CRP c-reactive protein.](https://researchreport.i-med.ac.at/wp-content/uploads/2023/09/Fig.-2-768x452.jpg)