
Peter Mayer Straße 1A
6020 Innsbruck
Fax: +43 512 9003 73200
Email: francesco.ferraguti@i-med.ac.at
Website: https://www.i-med.ac.at/pharmakologie/
Research year
Research Branch (ÖSTAT Classification)
3012
Keywords
anxiety disorders, Epileptogenesis, Fear learning, Feeding, Gene therapy, Interneurons, Metabotropic glutamate receptors, Neuropeptides, and Opioid receptors
Research Focus
- Characterisation of the neural networks underlying physiological and pathological fear/anxiety and identification of novel treatment strategies
- Interaction of hunger and fear with a specific focus on neuropeptide systems
- Aetiology and novel treatment of temporal lobe epilepsy
- Disease specific engineering of designer receptor gene therapies
General Facts
The Department of Pharmacology, established in 1886, is a centre of excellence in neuro and psychopharmacology and uses a variety of cutting-edge experimental approaches to address fundamental research questions related to the identification of novel molecular targets and the development of new therapeutic concepts for neuropsychiatric disorders.
The department provides pharmacology training to medical undergraduate and graduate students. An additional task of the institute is to contribute to the promotion of pharmacology and neuroscience within national and international societies. The Department of Pharmacology also provides independent drug and therapeutic information to doctors through the “Pharmainformation” bulletin and contributes to a variety of public bodies (e.g. the Ethics Committee of the Medical University of Innsbruck) involved in the evaluation of drug safety and development.
Research
Neural Circuits Underlying Emotional Behaviour
Francesco Ferraguti
The laboratory is interested primarily in understanding how emotional information is processed in the brain and in the roles of classical neurotransmitters (e.g. glutamate and GABA) and their receptors in anxiety and other negative emotions.
We have shown that associative learning, such as Pavlovian fear conditioning, elicits concerted structural and functional plasticity of amygdala GABAergic synapses. This process regulates both somatic and dendritic inhibition and may tune amygdala circuit responses to threats. Our more recent work has focused on a subclass of cortical inhibitory interneurons (IN) expressing vasoactive intestinal peptide (VIP). Taking advantage of recent developments in deep-brain calcium imaging and optogenetics, we have studied how their activity is modulated during associative learning as well as through salient stimuli and expectation. Moreover, using viral mono-transsynaptic tracing, we are elucidating the long-range connections of these interneurons in the basolateral amygdala and insular cortex of rodents.
One further important aim is to identify the neural circuits in which the pharmacological antagonism of the metabotropic glutamate 5 (mGlu5) receptor exerts its anxiolytic activity.
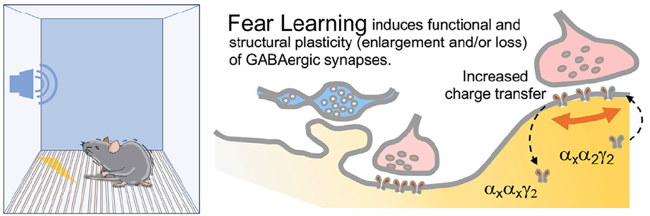
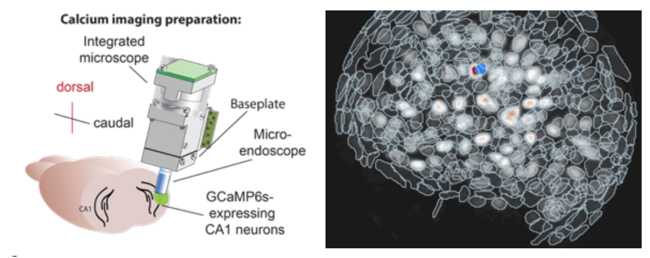
Opioid Systems in Epilepsy and Emotional Control
Christoph Schwarzer
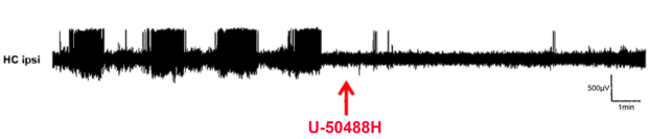
The laboratory investigates the role of the endogenous dynorphin/kappa opioid receptor (KOR) and enkephalin/delta opioid receptor (DOR) systems in epilepsy and epileptogenesis. It additionally aims to gain further insight into the functional neuroanatomy of the dynorphin/KOR in emotional control.
Epilepsy is one of the most common neurological diseases, which is incurable at present time. On average, 30% of patients do not achieve freedom from seizures through pharmacological treatment, rendering surgical removal of parts of the brain as ultimate solution. In the quest for novel treatment options, we investigate the role of the endogenous dynorphin / kappa opioid receptor (KOR) system in epilepsy and epileptogenesis. In recent years, we have provided evidence that the application of a KOR agonist during epileptogenesis reduces neurodegeneration and neurochemical alterations. Applying 4-channel in-vivo EEG combined with behavioural testing, we investigate G-protein-biased KOR agonists and AAV-based overexpression of dynorphin in the kainic acid model of temporal lobe epilepsy. In parallel with this, we study the role of hypoxic preconditioning as a potential neuroprotective and anti-epileptic treatment.
Moreover, we want to gain insight into the functional neuroanatomy of the dynorphin/KOR in emotional control. This is important in order to minimise the potential side-effects of KOR agonist treatment but also to understand the neurological basis of stress and stress-induced relapse in addiction.
Neuropeptides at the Crossroads of Fear and Hunger
Ramon Tasan
Avoiding danger and finding food are two intimately connected, life-sustaining phenomena that are organised in survival circuits and strongly modulated by emotions. Maladaptation within such circuits can induce dysregulated behaviour, which results in the development of feeding and anxiety disorders. Interestingly, neuropeptides are essential modulators of energy homeostasis and anxiety-related behaviours.
The laboratory investigates the role of neuropeptide transmitters in emotional behaviours related to fear and hunger. Neuropeptides are highly enriched in the extended amygdala, a brain area that controls emotional responses. There, neuropeptides act as essential modulators, which significantly shape synaptic function.
Through a multidisciplinary approach, we have demonstrated that several neuropeptides of the gut-brain axis are fundamentally involved in the modulation of fear and fear-extinction learning, an effect that is highly dependent on the homeostatic situation of an individual, emphasising the mutual interaction of survival circuits for fear and hunger. For instance, neuropeptide Y, which is released during states of hunger, has potent anxiolytic and fear-reducing properties, whereas neurokinin B, a neuropeptide in the tachykinin family, promotes fear. Our aim is to identify potential interaction points, where neuropeptides shape feeding and fear-related function.
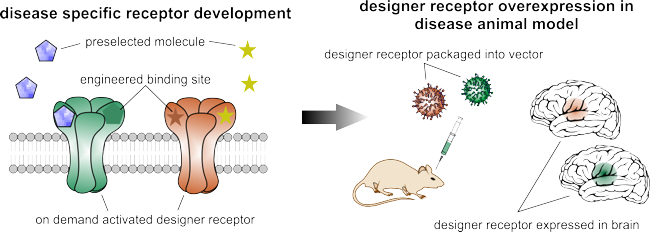
Disease-Specific Engineering of Designer Receptor Gene Therapies
Andreas Lieb
The main research aims of the laboratory are preclinical development and optimisation of gene therapies for neurological disorders, such as epilepsy, Parkinson’s disease, neuropathic pain, and multiple sclerosis.
Refractory epilepsy and Parkinson’s disease are among the most common neurological disorders, respectively with over 21 million and over 6 million patients worldwide. Despite huge efforts to investigate and develop novel treatment strategies, both diseases are still difficult to treat.
Multiple gene therapy options targeting Parkinson’s disease or refractory epilepsy have already been explored. However, all of them are limited by specific disadvantages, which we aim to overcome through disease-specific engineering of designer receptors.
In recent years, we have developed a biochemical autoregulatory gene therapy system that targets refractory epilepsy. This is activated only if the excitatory neurotransmitter glutamate rises pathologically and suppresses emerging seizures. In addition, we have identified a novel licensed drug that triggers the currently available designer receptor exclusively activated by designer drugs (DREADD). This drug exhibits a superior side-effect profile, which facilitates the translation of this technology.
Together, these major achievements highlight the fact that custom engineering of disease-specific designer receptors is indeed feasible and will help in the development of technologies with maximised therapy efficacy and minimal risk of side effects.
At present, we are developing new gene therapy strategies for the treatment of refractory epilepsy and enhancing existing technologies. In addition, we will expand the on-demand engineering of designer receptor gene therapies to other neurological diseases, such as Parkinson’s disease.
Pictures
Selected Publications
- Structural and functional remodeling of amygdala GABAergic synapses in associative fear learning. Kasugai Y., Vogel E., Hörtnagl H., Schönherr S., Paradiso E., Hauschild M., Göbel G., Milenkovic I., Peterschmitt Y., Tasan T., Sperk G., Shigemoto R., Sieghart W., Singewald N., Lüthi A., Ferraguti F. NEURON: 2019; 104(4):781-794.e4. doi: 10.1016/j.neuron.2019.08.013.
- Adaptive disinhibitory gating by VIP interneurons permits associative learning. Krabbe S., Paradiso E., D’Aquin S., Bitterman Y., Courtin J., Xu C., Yonehara K., Markovic M., Müller C., Eichlisberger T., Gründemann J., Ferraguti F., Lüthi A. Nature Neurosci.: 2019; 22(11):1834-1843. doi: 10.1038/s41593-019-0508-y.
- Increased anxiety-like behavior following circuit-specific catecholamine denervation in mice. Ferrazzo S., Gunduz-Cinar O., Stefanova N., Pollack G.A., Holmes A., Schmuckermair C., Ferraguti F. Neurobiol. Dis.: 2019; 125:55-66. doi: 10.1016/j.nbd.2019.01.009.
- Dynorphin-based “release on demand” gene therapy for drug-resistant temporal lobe epilepsy. Agostinho AS, Mietzsch M, Zangrandi L, Kmiec I, Mutti A, Kraus L, Fidzinski P, Schneider UC, Holtkamp M, Heilbronn R, Schwarzer C. EMBO Mol Med. 2019 Oct;11(10):e9963. doi: 10.15252/emmm.201809963.
- Functional characterization of novel bumetanide derivatives for epilepsy treatment. Auer T, Schreppel P, Erker T, Schwarzer C. Neuropharmacology. 2020 Jan 1;162:107754. doi: 10.1016/j.neuropharm.2019.107754.
- On the objectivity, reliability, and validity of deep learning enabled bioimage analyses. Segebarth D, Griebel M, Stein N, von Collenberg CR, Martin C, Fiedler D, Comeras LB, Sah A, Schoeffler V, Lüffe T, Dürr A, Gupta R, Sasi M, Lillesaar C, Lange MD, Tasan RO, Singewald N, Pape HC, Flath CM, Blum R. Elife. 2020 Oct 19;9:e59780. doi: 10.7554/eLife.59780.
- Amygdala NPY Circuits Promote the Development of Accelerated Obesity under Chronic Stress Conditions. Ip CK, Zhang L, Farzi A, Qi Y, Clarke I, Reed F, Shi YC, Enriquez R, Dayas C, Graham B, Begg D, Brüning JC, Lee NJ, Hernandez-Sanchez D, Gopalasingam G, Koller J, Tasan R, Sperk G, Herzog H. Cell Metab. 2019 Jul 2;30(1):111-128.e6. doi: 10.1016/j.cmet.2019.04.001.
- Olanzapine: A potent agonist at the hM4D(Gi) DREADD amenable to clinical translation of chemogenetics. Weston M., Kaserer T., Wu A., Mouravlev A., Carpenter J.C., Snowball A., Knauss S., von Schimmelmann M., During M.J., Lignani G., Schorge S., Young D., Kullmann D.M., Lieb A. Sci Adv.: 2019;5(4):eaaw1567. doi: 10.1126/sciadv.aaw1567.
Selection of Funding
- Bedeutung von NPY in Furcht und Essverhalten. FWF P 34320 Einzelprojekte. R. Tasan.
- Neurokinin B in emotionalen und metabolischen Prozessen. FWF P 33727 Einzelprojekte. R. Tasan.
- Bedeutung von Neurokinin B Neuronen im Bed Nucleus der Stria terminalis. FWF P 29952 Einzelprojekte. R. Tasan.
- Designer Rezeptor Gentherapie für die Parkinson’s Krankheit. FWF P 33222 Einzelprojekte. A. Lieb.
- Das Kappa Opioid System als Ziel zur Behandlung der TLE. FWF P 30592 Einzelprojekte. C. Schwarzer.
- Modulation des Kappa Opioid Systems in der TLE. FWF P 30430 Einzelprojekte. C. Schwarzer.
- Hippocampal circuits mediating the anxiolytic activity of mGlu5 receptors. OeAD TW 06/2020. F. Ferraguti
Collaborations
- Prof. Capogna M., Aarhus University, Copenhagen, Denmark.
- Prof. Harkany T., Center for Brain Research, University of Vienna, Vienna, Austria.
- Prof. Heilbronn R., Dept. Virology, Campus Benjamin Franklin, Charité – Medical School, Berlin, Germany.
- Prof. Herzog H., Garvan Institute of Medical Research, Australia.
- Prof. Hildebrand P., University Leipzig, Leipzig, Germany
- Prof. Kullmann D.M., University College London, London, UK
- Prof. Lien C-C., National Yang-Ming University, Taipei, Taiwan.
- Prof. Lüthi A., Friedrich Miescher Institute, Basel, Switzerland.
- Prof. Liu-Chen L-Y., Temple University, Philadelphia, USA