Innrain 80
6020 Innsbruck
Fax: +43 512 9003 73960
Email: andreas.villunger@i-med.ac.at
Website: http://www.villungerlab.com/
Research year
Research Branch (ÖSTAT Classification)
301105, 301108, 301902, 301904
Keywords
Cell Death, Haematopoiesis, Immunology, Inflammation, Non-coding RNAs, and Tumour Biology
Research Focus
With their co-workers, IDI investigators explore basic mechanisms of immune cell development and function with an emphasis on cell death – cell cycle crosstalk, innate and adaptive immunity, microRNAs and steroid hormones. In addition, we are interested in studying general principles of cellular transformation, focusing on the role of BCL2-regulated cell death and the p53 signalling network as barrier against malignant disease. For additional details, please see https://www.villungerlab.com
General Facts
The Institute is part of the Biocenter (https://biocenter.i-med.ac.at ) and organised in four research groups, under the leadership of the Institute Director Andreas Villunger and associate professors Joel Riley, Verena Labi and Sebastian Herzog. The local resources and infrastructure available include ample modern lab space, preclinical models of malignant disease, immune-deficiency and autoimmunity, S2-tissue culture for lenti-or retroviral transduction of model cell lines, histology support, cell sorting (BD FACS_ARIAIII), multi-colour flow cytometry (BD FACS-LSR-Fortessa), live-cell imaging (IncuCyte) and a CRISPR/Cas9-based screening platform. The Center of Molecular Medicine (CeMM) of the Austrian Academy of Science in Vienna, where Andreas Villunger holds an adjunct PI position, also provides swift access to PLACEBO drug-screening and the biomedical sequencing facility (BSF) for RNAseq studies (https://cemm.at).
All faculty members contribute to teaching and training of students enrolled in human medicine of life-sciences at bachelor and master levels. A structured PhD program secures a first-class training environment for the PhD students of our institute (https://phd-school.i-med.ac.at/). Scientific exchange is guaranteed at various levels, including regular group meetings, institute and department seminars, as well as international conference participations.
Research
The Cell Death Signalling Lab
Andreas Villunger
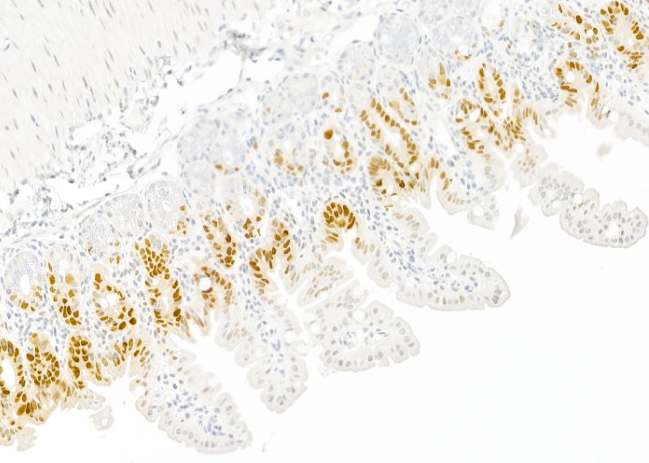
Within the Cell Death Signalling lab under the leadership of the Institute Director, Andreas Villunger, we are currently exploring the cross talk of the cell death and the cell cycle machinery. Thereby, we focus on understanding signalling events that define thresholds for cell death or survival following mitotic errors. Two aspects concern us. How are mitotic errors communicated to alert the immune system and how is p53, a major tumour suppressor, engaged in this context, e.g. in response to centrosomal abnormalities. Within these studies, we have shown that PIDD1, sitting at the centrosome in healthy cells (Figure 1), can trigger sterile inflammation when centrosomes accumulate.
Moreover, we investigate the consequences of centrosome amplification for tissue homeostasis. Overexpression of Polo-like Kinase 4 (PLK4) leads to centrosome amplification and engages the PIDDosome that drives p53 activation and expression of the cell cycle regulator, p21 (Figure 2). It is believed that this process limits transformation but may also be the desired outcome during normal organ development, as exemplified in the liver or heart.
The Cell Death & Inflammation Lab
Joel Riley
Immunogenicity of cell death
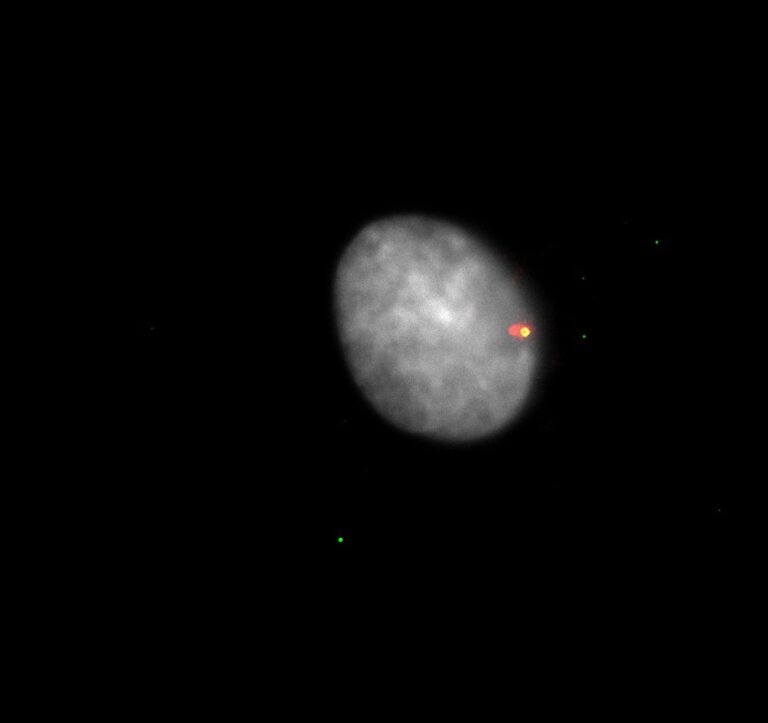
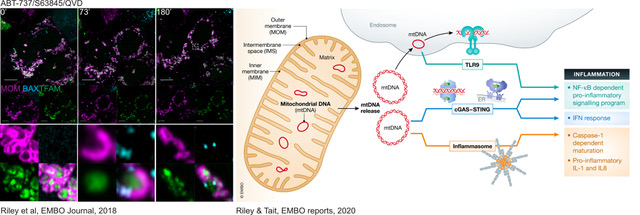
Cell death, and in particular programmed cell death modalities such as apoptosis, have long been considered to be immune-silent, as they do not trigger any immune response. However, it is now becoming clear that apoptosis can in fact be highly pro-inflammatory under some circumstances. We have shown that apoptosis triggers a potent immune response when caspases are inhibited (genetically or pharmacologically). This is in part due to the release of mitochondrial DNA into the cytoplasm, where it is sensed by innate immune sensors such as cGAS-STING, initiating a potent type I interferon response (Figure 3). The consequences of mtDNA release during cell death is under active investigation, and work from us and others suggests that it could have a huge impact on the pathology of various diseases.
Oncogenic cell death signalling
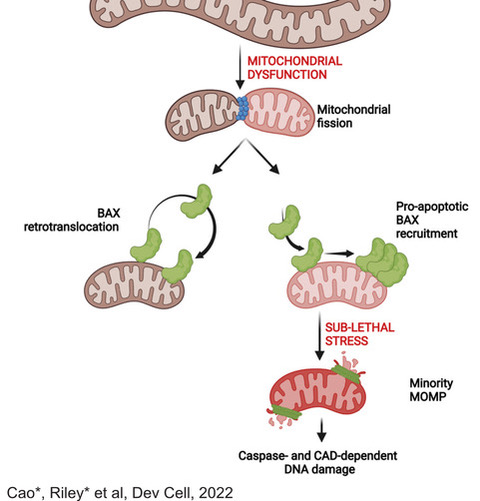
A hallmark of cancer is the ability to evade cell death. However, we have shown that low level activation of the apoptotic machinery can in fact be oncogenic, rather than anti-tumour. Using super-resolution imaging, we have been able to demonstrate that fragmented, dysfunctional mitochondria accumulate pro-apoptotic proteins on their membranes, allowing a limited, but detectable activation of caspases. Importantly, this level of caspase activation is not sufficient to cause cell death, but does cause DNA damage, which can be oncogenic (Figure 4). Current research in the lab is aimed at further understanding how this process contributes to oncogenesis, particularly in B cell lymphomagenesis.
The Experimental Immunology Lab
Verena Labi
Within the Experimental Immunology lab run by the deputy institute head, Verena Labi, we study molecular control mechanisms of cell fitness and fate decisions. Through genetic cell culture and mouse models, we aim to understand normal cell differentiation in comparison to pathological conditions.
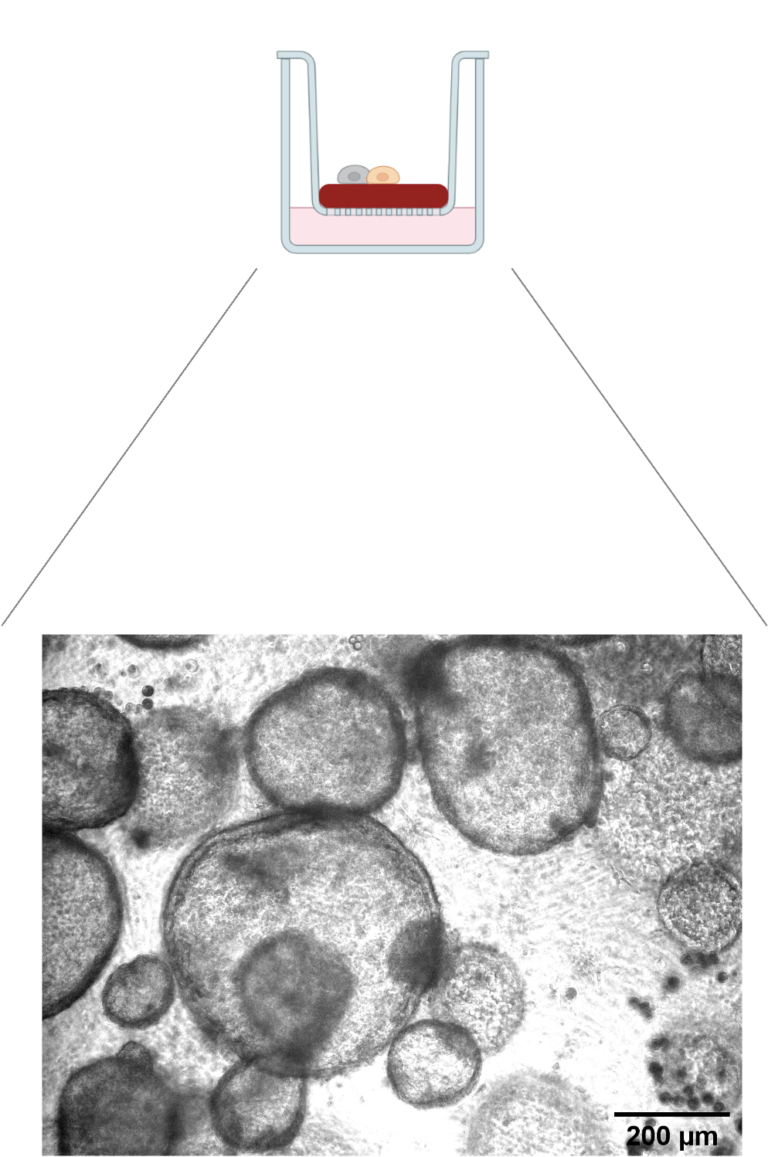
We demonstrated previously that the miR-17-92 microRNAs prevent cell death in the developing lung via repression of the pro-apoptotic BIM protein. Following this line of research, we have established an in vitro lung organoid culture system (Figure 5) to test whether this molecular mechanism, vital for proper lung development, fosters cancer in the adult lung.
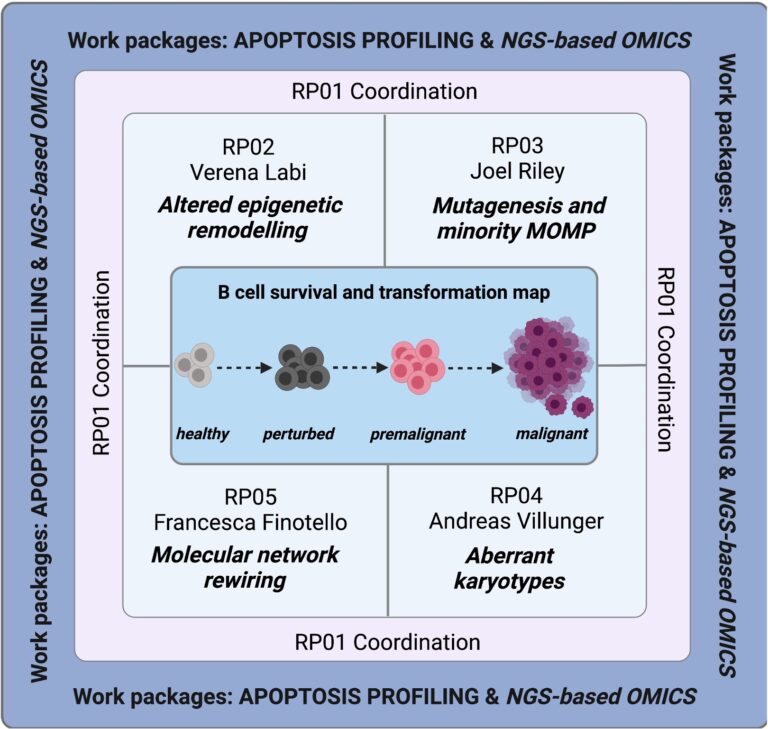
A second line of research is based on our finding that the epigenetic TET enzymes serve to establish and adapt transcriptional identity programs during B cell differentiation and activation. Notably, the TET enzymes ensure optimal humoral immunity while preventing pathologies such as immunodeficiency, autoimmunity and lymphomagenesis. Thus, we were recently able to secure further funding for the elucidation of the tumour suppressor mechanisms of TET2 in B cells at the molecular and cellular level. Our project is embedded in a research group with A. Villunger and J. Riley from our Institute, as well as Francesca Finotello from the UIBK. Together, we aim to develop a road map to the molecular rewiring of cell death networks in B cell transformation (Figure 6).
The Molecular Gene Regulation Lab
Sebastian Herzog
Initially considered as “junk” DNA, it is now clear that the non-protein coding part of the human genome, which comprises about 98 % of the ~3·109 DNA bases, is extensively transcribed and gives rise to numerous non-coding RNAs including miRNAs, small non-coding RNAs with a critical role in post-transcriptional gene regulation. Our general objective is to understand how microRNAs regulate gene expression on the molecular level and to what extend this regulation impacts on cellular function.
MicroRNAs in B lymphocytes
In this section, we aim to decipher how miRNAs impact on the sensitive processes that control B cell development and activation, which jointly ensure the establishment of a competent humoral immune system. We approach this topic with a two-pronged strategy, using gain-of-function as well as loss-of-function experiments and in vivo models to study transforming and physiologically relevant miRNAs.
MiRNA biogenesis
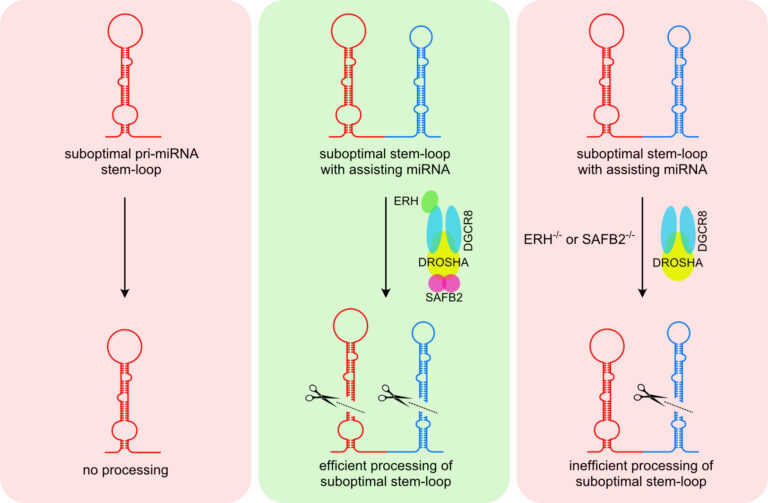
In canonical miRNA biogenesis, miRNA genes give rise to long primary transcripts (pri-miRNAs) that are characterised by one or several stem-loop structures in which the mature miRNAs are embedded. These stem-loop structures are recognised and cleaved by the nuclear Microprocessor, composed of the RNase DROSHA and its co-factor DGCR8. While most pri-miRNA is well defined by structural and sequence features, it is still not fully understood how cells can discriminate miRNA-like stem-loop structures from authentic primary miRNAs and how accessory proteins beyond the core components shape the mature miRNA transcriptome.
In this context, we have recently characterised a novel mechanism called cluster assistance (Figure 7) that significantly broadens the Microprocessor substrate specificity. Here, a bona fide pri-miRNA stem-loop structure functions as a cis-regulatory element that can license or enhance the processing of neighbouring suboptimal pri-miRNAs within polycistronic clusters. However, we are far from understanding this phenomenon, and our current efforts aim to unravel the mechanisms underlying cluster assistance and miRNA biogenesis in general.
Pictures
Selected Publications
Weiss JG, Gallob F, Rieder P, Villunger A. Apoptosis as a Barrier against CIN and Aneuploidy. Cancers (Basel). 2022 Dec 21;15(1):30. doi: 10.3390/cancers15010030. PMID: 36612027; PMCID: PMC9817872.
Kim JY, Wang LQ, Sladky VC, Oh TG, Liu J, Trinh K, Eichin F, Downes M, Hosseini M, Jacotot ED, Evans RM, Villunger A, Karin M. PIDDosome-SCAP crosstalk controls high-fructose-diet-dependent transition from simple steatosis to steatohepatitis. Cell Metab. 2022 Oct 4;34(10):1548-1560.e6. doi: 10.1016/j.cmet.2022.08.005. Epub 2022 Aug 29. PMID: 36041455; PMCID: PMC9547947.
Lohmüller M, Roeck BF, Szabo TG, Schapfl MA, Pegka F, Herzog S, Villunger A, Schuler F. The SKP2-p27 axis defines susceptibility to cell death upon CHK1 inhibition. Mol Oncol. 2022 Aug;16(15):2771-2787. doi: 10.1002/1878-0261.13264. Epub 2022 Jul 7. PMID: 35673965; PMCID: PMC9348596.
Weiler ES, Szabo TG, Garcia-Carpio I, Villunger A. PIDD1 in cell cycle control, sterile inflammation and cell death. Biochem Soc Trans. 2022 Apr 29;50(2):813-824. doi: 10.1042/BST20211186. PMID: 35343572; PMCID: PMC9162469.
Mitochondrial inner membrane permeabilisation enables mtDNA release during apoptosis. Riley JS, Quarato G, Cloix C, Lopez J, O’Prey J, Pearson M, Chapman J, Sesaki H, Carlin LM, Passos JF, Wheeler AP, Oberst A, Ryan KM, Tait SW. EMBO J. 2018. Sep 3;37(17): e99238. PMID 30049712
Mitochondrial dynamics regulate genome stability via control of caspase-dependent DNA damage. Cao K*, Riley JS*, Heilig R, Montes-Gomez, Vringer E, Berthenet K, Cloix C, Elmasry Y, Spiller DG, Ichim G, Campbell KJ, Gilmore AP, Tait SW. Dev Cell. 2022. May 23;57(10): 1211-1225. PMID 3544090
MCL-1 and BCL-XL: blood brothers. Erlacher M, Labi V. Blood. 2021 Apr 8;137(14):1850-1851. doi: 10.1182/blood.2020010569. PMID: 33830190
miR-142 favors naïve B cell residence in peripheral lymph nodes. Hagen M, Chakraborty T, Olson WJ, Heitz M, Hermann-Kleiter N, Kimpel J, Jenewein B, Pertoll J, Labi V, Rajewsky K, Derudder E. Front Immunol. 2022 Nov 10;13:847415. doi: 10.3389/fimmu.2022.847415. eCollection 2022. PMID: 36439112
SAFB2 Enables the Processing of Suboptimal Stem-Loop Structures in Clustered Primary miRNA Transcripts. Hutter K, Lohmüller M, Jukic A, Eichin F, Avci S, Labi V, Szabo TG, Hoser SM, Hüttenhofer A, Villunger A, Herzog S. Mol Cell. 2020 Jun 4;78(5):876-889.e6; doi: 10.1016/j.molcel.2020.05.011. PMID: 32502422.
The miR-15a/16-1 and miR-15b/16-2 clusters regulate early B cell development by limiting IL-7 receptor expression. Hutter K, Rülicke T, Szabo TG, Andersen L, Villunger A, Herzog S. Front. Immunol. 2022 13:967914. doi: 10.3389/fimmu.2022.967914. PMID: 36110849. PMCID: PMC9469637.
The miR-26 family regulates early B cell development and transformation. Hutter K, Lindner SE, Kurschat C, Rülicke T, Villunger A and Herzog S. The Life Sci Alliance 2022 Apr 22;5(8):e202101303; doi: 10.26508/lsa.202101303. PMID: 35459737. PMCID: PMC9034462.
Selection of Funding
European Research Council: ERC_AdG – POLICE
FWF: Research Group “BEAT-IT” – BCL-2 Network Adaptations in B Cell Transformation
FWF/GCAR: Cyanobacterial Metabolites for Cancer Therapy
FWF: Research Group “BEAT-IT” – BCL-2 Network Adaptations in B Cell Transformation
TWF: Using advanced microscopy to understand cell death
FWF: Coordinator Research Group “BEAT-IT” – BCL-2 Network Adaptations in B Cell Transformation
Collaborations
Alain de Bruin, Utrecht University, The Netherlands
Floris Foijer, ERIBA, Groningen, The Netherlands
Ana Garcia-Saez, University of Cologne, Germany
Luca Fava, CIBIO, Trento, Italy
Klaus Rajewsky, Max-Delbrück Center for Molecular Medicine, Berlin, Germany
Mir-Farzin Mashreghi, DRFZ, Berlin, Germany
Miriam Erlacher, Univ. Hospital, Freiburg, Germany
Yunsun Nam, University of Texas – Southwestern Medical Center, USA