Peter Mayr Str. 1a
6020 Innsbruck
Fax: +43 [0) 512 9003 73518
Email: gottfried.baier@i-med.ac.at
Website: https://www.i-med.ac.at/cell-genetics/
Research Branch (ÖSTAT Classification)
301902, 302055, 301303
Keywords
cancer immunity, T cell effector/memory functions, and T lymphocyte signaling
Research Focus
The fundamental goal of our work is to understand the selective functions of defined signaling pathways in CD4+ and CD8+ T lymphocytes and to use this information to develop strategies to manipulate the immune response to overcome immunosuppression in the context of cancer immunotherapy approaches. The cell genetics team has expertise in mouse genetics, in the investigation of effector/memory T cell differentiation and, in particular, molecular signaling processes through the use of hypothesis-driven mechanistic studies as well as unbiased CRISPR/Cas9-based genetic screens and RNA sequencing.
General Facts
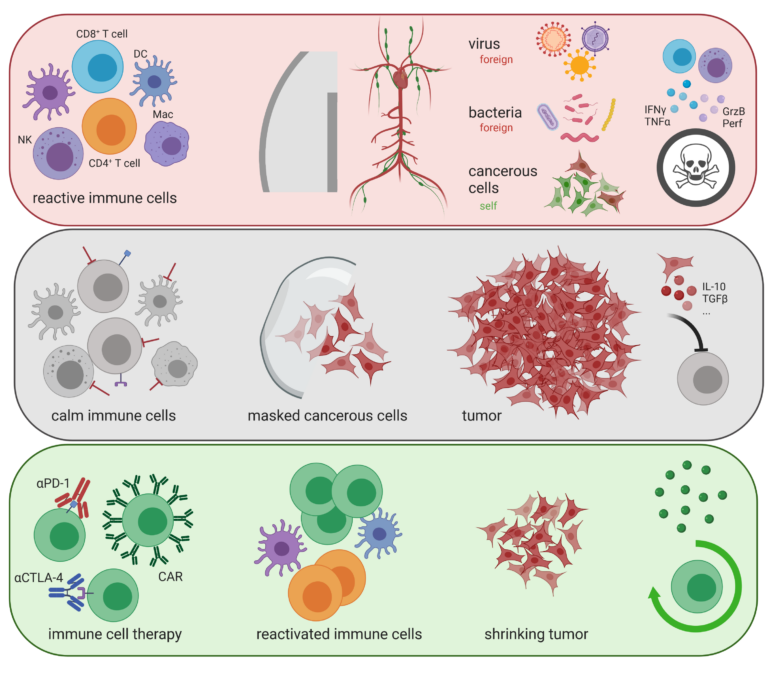
The most successful strategies for cancer immunotherapy have focused on targeting T cell surface receptors such as CTLA-4 and/or PD-1 with recombinant antibodies, which has been a game changer for cancer treatment. However, a significant subset of patients still fails to respond to these regimens, and even fewer patients are potentially cured.
Using germline gene ablation as well as CRISPR/Cas9-mediated acute gene mutagenesis, the orphan nuclear receptor NR2F6 (nuclear receptor subfamily 2 group F member 6, also known as Ear-2) has been firmly characterized as one such intracellular immune checkpoint in the effector T cell compartment capable of improving antitumor immunotherapy responses, especially in combination with CTLA-4 and PD-1. Current preclinical experimental evidence defines key protein-protein and protein-DNA interactions that strongly validate the immune function of lymphatic NR2F6 in tumor immune evasion. Remarkably, the strategy of NR2F6 checkpoint targeting therefore appears particularly capable of improving the efficacy and broadening the applicability of cancer immunotherapy regimens, and thus has the potential to strengthen the immune system of tumor patients in the near future.
Research
Research focus: Mechanistic understanding of T cell effector function in host protective tumor immunity
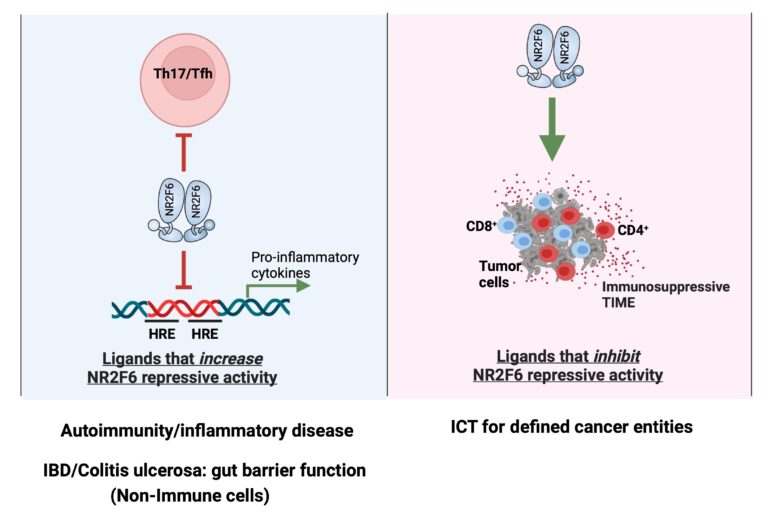
The Cell Genetics team was the first to identify the T lymphocyte intrinsic PKCtheta/NR2F6/Cbl-b axis as an essential signaling node governing the complex host-tumor interactions at the interface between inflammation and cancer. Modulation of this pathway renders effector T cells capable of rejecting otherwise lethal tumor loads and their metastases in preclinical cancer model systems. Our research aims to elucidate inter- and intracellular mechanisms that remodel the immune context to allow for superior tumor rejection (Fig. 1).
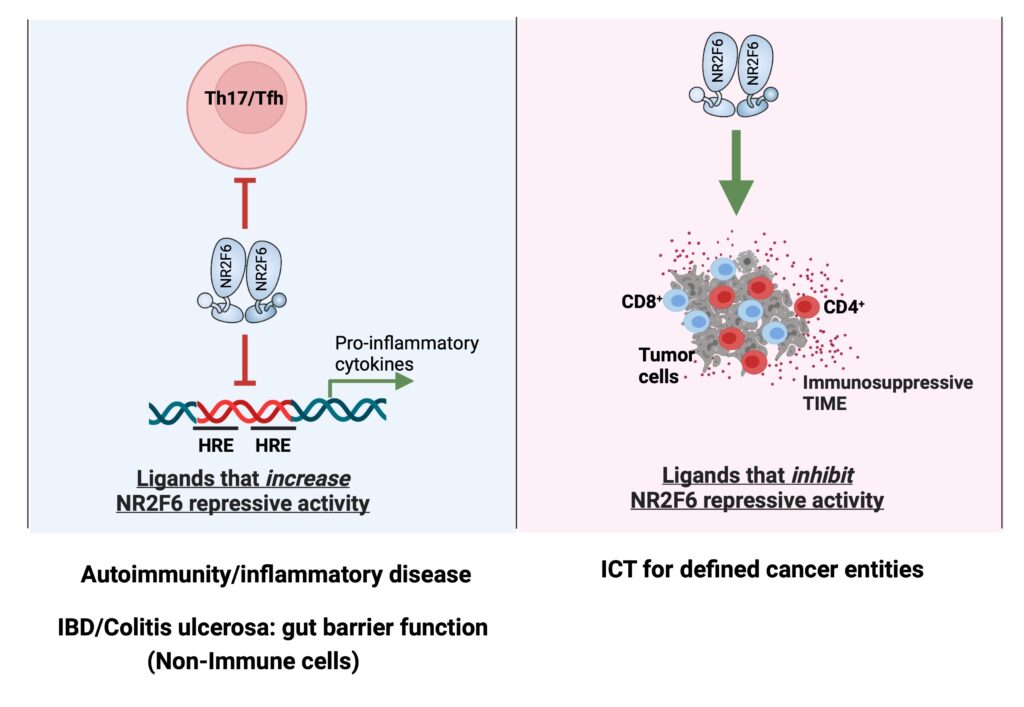
NR2F6 forms homo- and heterodimers (e.g. with RXRs) and acts as a transcriptional repressor or activator in a promoter context-dependent manner. Although natural ligands for NR2F6 are currently unknown, the potential druggability of its ligand binding domain (LBD) for a “small molecule modulator” could provide a rational mechanistic basis for targeted manipulation of NR2F6 as an alternative immune checkpoint in T cells (ICT, immune checkpoint therapy).
AG Gruber: Role of the E3 ubiquitin ligase Cbl-b in cancer immunity
Adoptive transfer of genetically silenced Cbl-b-deficient CD8+ cytotoxic T lymphocytes significantly enhances anti-tumor efficacy in immunocompetent recipient mice. The goal of the translational research collaboration is to elucidate the underlying mechanisms in immune cells by which the Cbl-b pathway attenuates their effector responses in an immunosuppressive tumor environment (Fig. 2).
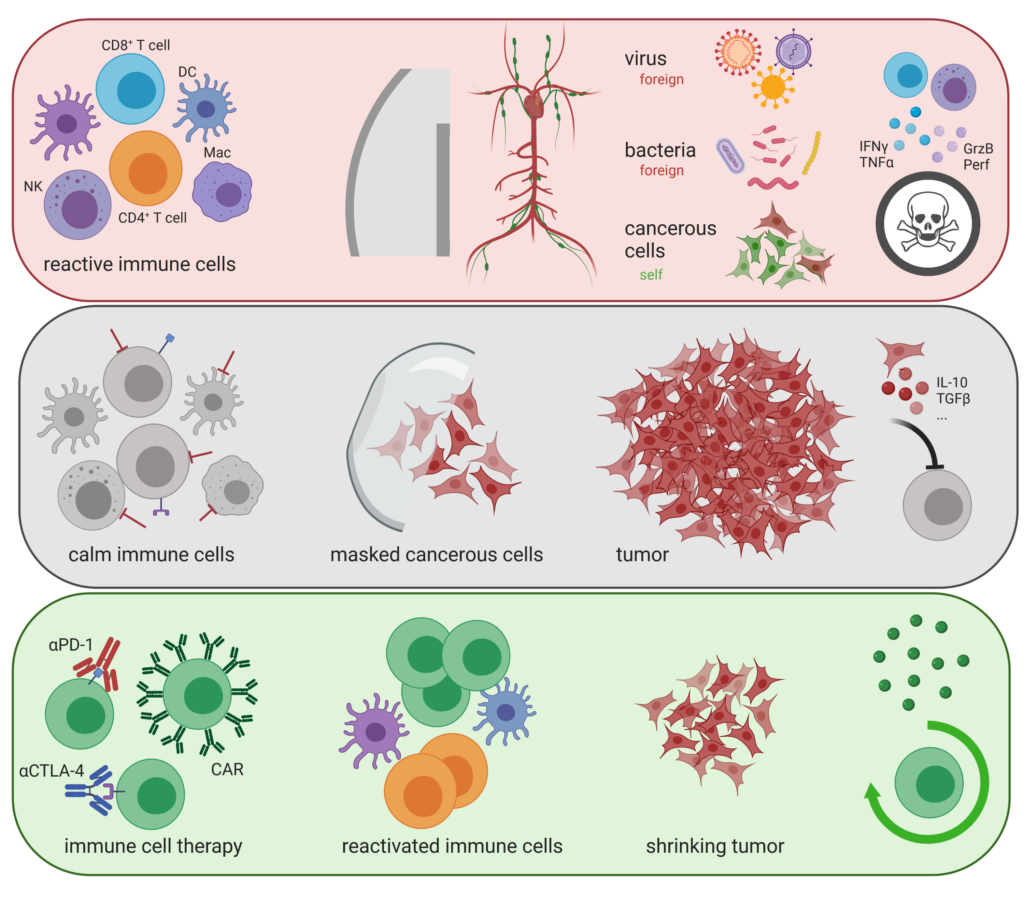
AG Klepsch: Identification of new pathways critical in cancer immunity
The future of immuno-oncology therapy development lies in combination regimens. Using both rational approaches and CRISPR/Cas9 pooled library screens in mice, we are exploring novel target candidates essential for the physiology and pathophysiology of T lymphocyte biology.
AG Bellaire-Siegmund: Role of selected protein kinases in adaptive immunity
The underlying goal is to understand the selective functions of defined signal transduction pathways in CD4+ and CD8+ T lymphocytes mediated by protein kinases such as PKC and PKD.
AG Thuille: Role of metabolic regulation in T cell biology
The aim is to decipher the biochemical processes of metabolic regulation that integrate key signals received from antigen, cytokine and integrin-receptors as well as inhibitory receptors in primary T cells.
AG Kleiter: Role of NR2F6 in immune responses to infection
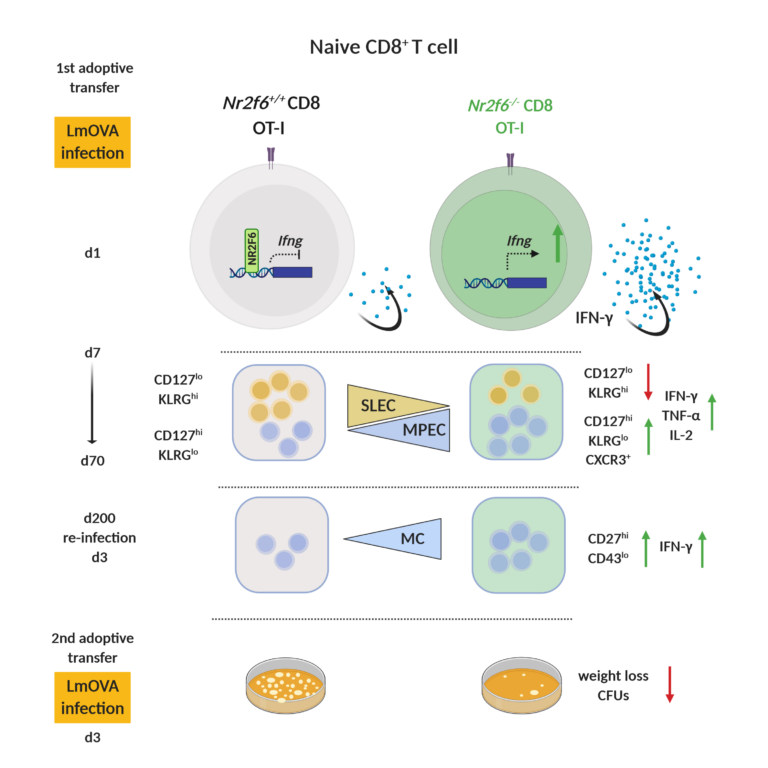
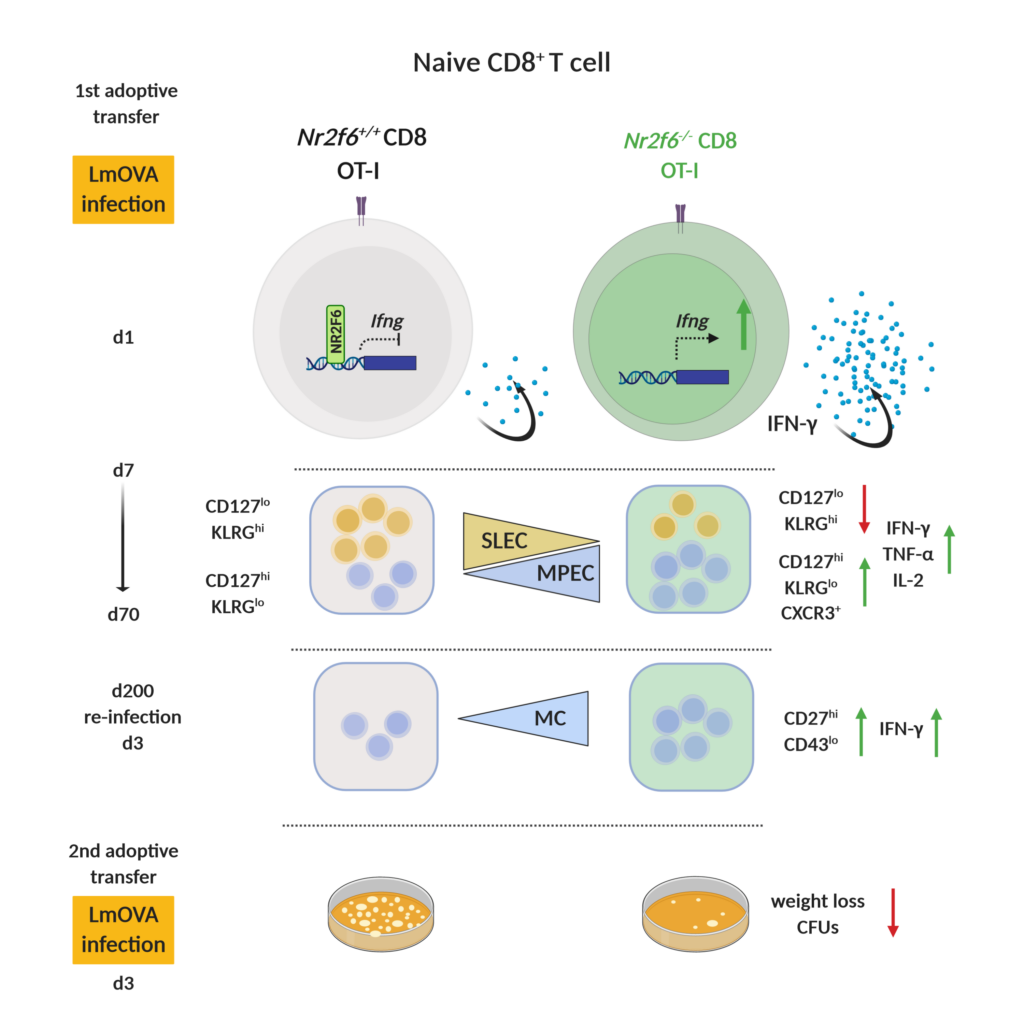
Immunological memory formation represents a cardinal feature of adaptive immunity. Research using molecular and cellular analyses of the nuclear receptor network defines NR2F6 as one signal transducer that regulates antigen-specific CD8+ T cell memory formation during bacterial infection with Listeria monocytogenes (Fig. 4).
Pictures
Selected Publications
Kim H, Feng Y, Murad R, Pozniak J, Pelz C, Chen Y, Dalal B, Sears R, Sergienko E, Jackson M, Ruppin E, Herlyn M, Harris C, Marine JC, Klepsch V, Baier G, Ronai ZA. Melanoma-intrinsic NR2F6 activity regulates antitumor immunity. Sci Adv. 2023 Jul 7;9(27):eadf6621. doi: 10.1126/sciadv.adf6621. Epub 2023 Jul 5. PMID: 37406115.
Koutník J, Leitges M, Siegmund K. T cell-intrinsic protein kinase D3 is dispensable for the cells’ activation. Front Immunol. 2022 Nov 17;13:1049033. doi: 10.3389/fimmu.2022.1049033.PMID: 36466811; PMCID: PMC9713823.
Koutník J, Klepsch V, Pommermayr M, Thuille N, Baier G, Siegmund K. A MLR-Based Approach to Analyze Regulators of T Lymphocyte Activation In Vivo. Int J Mol Sci. 2022 May 10;23(10):5337. doi: 10.3390/ijms23105337. PMID: 35628145; PMCID: PMC9140849
Schanz O, Cornez I, Yajnanarayana SP, David FS, Peer S, Gruber T, Krawitz P, Brossart P, Heine A, Landsberg J, Baier G, Wolf D. Tumor rejection in Cblb-/- mice depends on IL-9 and Th9 cells. J Immunother Cancer. 2021 Jul;9(7):e002889. doi: 10.1136/jitc-2021-002889. PMID: 34272310;PMCID: PMC8287598.
Jakic B, Olson WJ, Siegmund K, Klepsch V, Kimpel J, Labi V, Zehn D, Baier G, Hermann-Kleiter N. Loss of the orphan nuclear receptor NR2F6 enhances CD8+ T cell memory via IFN-γ. Cell Death Dis. 2021 Feb 15;12(2):187. doi: 10.1038/s41419-021-03470-9. PMID: 33589606; PMCID: PMC7884426
Selection of Funding
- ERC Advanced grant # 786462: Host Protective Engineering of Cancer Immunity, Acronym: HOPE (Coord. Gottfried Baier)
- PhD program in Molecular Cell Biology and Disease, Austrian Science Funds (FWF) DK-MCBD (Natascha Kleiter)
- Therapie zerebraler Malaria mit Adenosin2a Rezeptor Blockade, Austrian Science Funds (FWF) TAI 80 (Karin Albrecht-Schgör)
- Sox macht den Unterschied, Austrian Science Funds (FWF) TAI 88 (Kerstin Bellaire-Siegmund)
- Die Rolle der Proteinkinase D in T Zellen, Austrian Science Funds (FWF) P34368 (Kerstin Bellaire-Siegmund)
- Darmbakterien manipulieren Immunantwort gegen Krebs, Austrian Science Funds (FWF) T-1292 Firnberg-Programm (Victoria Klepsch)
- ERC Proof of concept grant, CAR-T-uning: Tuning Chimeric Antigen T Cell (CAR-T) therapy to lung cancer Acronym: CAR-T(uning) (Coord. Gottfried Baier)
- Potentiating CD8+ CAR-T cells by targeting of the lymphatic PKD3 pathway, ÖAW’s DOC research scholarship (Jiri Koutnik)
- Extension of CD8 CAR-T cell therapy success by CBLB pathway blockade; Acronym: EXCEL-T, FFG Bridge #49465145; together with invIOs; Vienna (Coord. Gottfried Baier)
Collaborations
Jürgen Wagner; Novartis Pharma, Basel, Switzerland
Dietmar Zehn, Chair of Immunology, TU Munich, Germany
Noah Isakov, Ben Gurion University of the Negev, Israel
Wallace Langdon, University of Western Australia, Perth, AUS
Shoji Yamamoto, Daiichi Sankyo Ltd., Tokyo, Japan
Michael Leitges, Memorial University of Newfoundland, St. John`s, Canada