Anichstraße 35
6020 Innsbruck
Fax: +43 (0)50 504 67 24811
Email: Wolfgang.Horninger@i-med.ac.at
Website: https://uro-innsbruck.tirol-kliniken.at/
Research year
Research Branch (ÖSTAT Classification)
302055, 302086, 302091
Keywords
bladder cancer, cancer drugs, Immunology, and prostate cancer
Research Focus
Our research is focused on the fundamental research and clinical aspects of common urological cancers, particularly prostate, bladder, and renal cancer.
We are committed to identifying biomarkers that enhance diagnosis and improve the monitoring of therapeutic success. We also investigate mechanisms of therapy resistance and explore novel drug targets for the treatment of urological malignancies.
General Facts
The Department of Urology at the Medical University of Innsbruck is a renowned institution. It is dedicated to patient care and scientific research. It offers a full range of diagnostic and therapeutic services for urological diseases, including prostate, bladder, and kidney cancer, as well as functional urological disorders.
A particular focus is placed on translational research, with close links to clinical practice. In this context, specialised research laboratories play a key role:
- Section of Experimental Urology (Head: Univ.-Prof. Dr. Zoran Culig)
Research focus: Mechanisms of cancer progression, therapy resistance, steroid receptors and novel therapeutic targets.
Contact: Tel. +43 (0)512/504-24717, Email: zoran.culig@i-med.ac.at - Laboratory for Immunology and Uro-Oncology (Head: Univ.-Prof. Dr. Martin Thurnher)
Research focus: Tumour immunology, immunotherapeutic approaches and interactions between tumour cells and the immune system.
Contact: Tel. +43 (0)512/504-24867, Email: martin.thurnher@i-med.ac.at - Professorship for Translational Prostate Cancer Therapy Research (Head Univ. Prof. Dr. Isabel Heidegger)
Research focus: Tumour microenvironment of prostate cancer, particularly focusing on the identification of new biomarkers and therapeutic target structures.
Contact: Email: isabel.heidegger@i-med.ac.at
The department includes highly specialised inpatient units, a day clinic and various expert consultations, including andrology, oncology, prostate diseases, and paediatric urology. The Department of Urology at Innsbruck is a world leader in urology, integrating clinical practice and research to deliver innovative diagnostic and therapeutic approaches.
Research
Tumour immunology and immunotherapy
Thurnher, D. Klaver
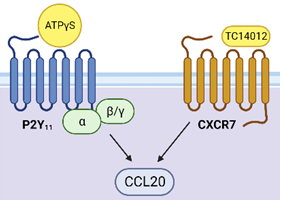
P2Y11 – chemokine receptor crosstalk (BioRender, Copyright: Thurnher/Klaver)
The Tumour Immunology Unit examines the interaction between developing tumours, such as renal cell carcinoma, and the immune system. It is clear that a complex chemokine/chemokine receptor network orchestrates the multi-faceted interactions between growing tumour tumours and infiltrating immune cells. Our study of tumour-immune cell interactions in situ is complemented by studies of differentiating human macrophages in vitro. This provides a framework for a better understanding of the development of tumour-associated macrophages that fuel pathogenic inflammation. In this context, we also examine the human-specific G protein-coupled ATP receptor P2Y11, which regulates chemokine receptor expression in a highly selective manner. Finally, we examine macrophage activation by Bacillus Calmette-Guérin (BCG), the most efficient adjuvant therapy for high-risk, non-muscle-invasive bladder cancer (NMIBC). Our overriding goal is to translate our findings and knowledge into improved therapies and measures to minimise side effects.
Unveiling three distinct molecular clusters in urothelial carcinoma: a roadmap for clinical precision
Renate Pichler, Nils C. H. van Creij
Whole-transcriptome profiling has delivered the first comprehensive understanding of the molecular heterogeneity in urothelial cancer (UC). With the aim to introduce personalised medicine, previous studies have successfully divided UC into different molecular clusters with the aim of introducing personalised medicine. The consensus was clear: biological classes should be prioritised over clinical classes. This complexity has thus far hindered their integration into guideline recommendations and routine clinical practice. This is a result of the complexity of the data required for molecular classification, as well as the fact that most molecular classification schemes were developed and validated only in muscle-invasive bladder cancer. The aim of the current project is to establish a simple, unique, clinically applicable molecular classification scheme of urothelial cancer. This will be based on whole transcriptome and proteome data with clinical implications for prognosis and therapy. The result will be a broader clinical applicability to non-muscle invasive bladder cancer as well as to muscle-invasive bladder cancer. We assigned single-cell RNA sequencing data (scRNAseq), FFPE urothelial cancer samples and publicly available UC cell lines to the different UC clusters. This was achieved by comparing pharmacological, metabolic and biological characteristics. This provides a comprehensive understanding of the complex biology of UC through a multifaceted methodology. Our new insights will pave the way for the development of more effective therapies for each cluster within the context of personalised medicine in UC.
Personalised urothelial cancer therapy proposing novel cluster-specific therapeutic agents
Renate Pichler, Nils C. H. van Creij
In this follow-up project, we will test cluster-specific novel therapeutic targets (EGFR inhibitors, PARP inhibitors, MAPk inhibitors….) in vitro in different cell lines and ex vivo in cluster-specific UC organoids. The aim is to develop more efficient (combination) therapies, particularly for the more aggressive subtypes, and thereby improve the prognosis.
LifeBoost: Comprehensive, networked and holistic care improving the quality of life during immunotherapy through evidence-based integrative services
Renate Pichler, Bernhard Holzner, Jens Lehmann
The introduction of immunotherapy revolutionised the therapeutic landscape for many immunogenic tumour entities, including bladder cancer. The occurrence of immunotherapy-associated side effects leads to treatment pauses or discontinuation of therapy, which leads to a deterioration in quality of life and patient compliance. The aim of the LifeBoost project is to expand the concept of the Comprehensive Cancer Centre (CCC) to include an integrative and anthropocentric supportive care approach with the creation of multidisciplinary and multimodal, digital supportive services for patients undergoing immunotherapy. This supportive program includes the areas of nutrition, exercise, music education and psycho-oncology. The existing digital platform of the Austrian Cancer Aid supports the implementation of the project and enables a standardised recording of quality of life through PROMs, as well as a systematic content analysis. In summary, LifeBoost is characterised by the bundling of different expertise and the use of existing resources, creating valuable synergies. We actively involve patients to deliver personalised care that addresses their medical, emotional and social needs.
Adjuvant treatment selection in urothelial cancer after radical cystectomy based on circulating tumour cells (ATUC-CTC)
Felizian Lackner, Antonia Partl, Gerald Klinglmair, Renate Pichler
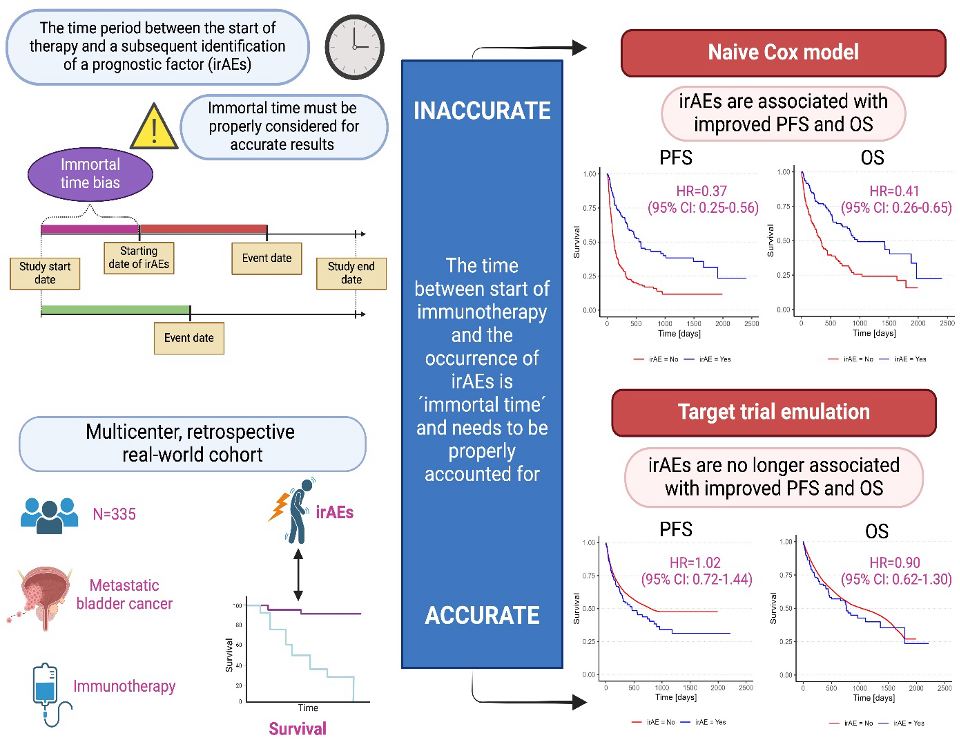
Radical cystectomy (RC) with pelvic lymph node dissection, is the standard treatment for muscle-invasive bladder cancer. However, nearly 50% of patients experience recurrence. The CheckMate 274 study states that “high-risk” patients (ypT2–T4a or nodal-positive [ypN+] in those treated with neoadjuvant chemotherapy [NAC]; pT3–T4a or pN+ in patients without NAC) should receive adjuvant nivolumab when tumour programmed death ligand 1 (PD-L1) expression level is ³ 1%. However, PD-L1 has limitations as a prognostic marker in the adjuvant setting, as shown by discordant disease-free survival data from both the CheckMate 274 and AMBASSADOR trial. Liquid biopsy may offer a more sensitive marker for the detection of micrometastases post-RC. The IMvigor010 study found that only 37% of “high-risk” patients were ctDNA positive and benefited from adjuvant immunotherapy with atezolizumab. Our study identifies circulating tumour cells (CTCs) as a biomarker to determine which MIBC patients will benefit most from adjuvant immunotherapy. This optimises treatment, reduces toxicities as well as healthcare costs.
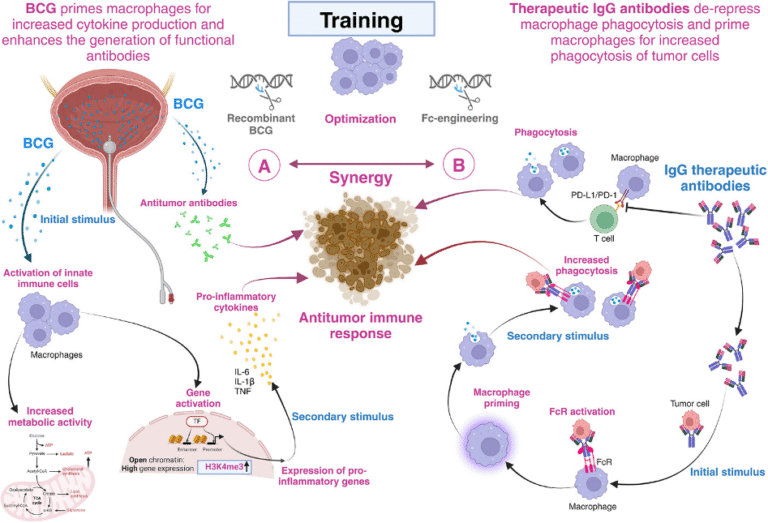
Training the synergy between Bacillus Calmette-Guérin and immune checkpoint-blocking antibodies in bladder cancer
Renate Pichler, Martin Thurnher
Fifty years after the introduction of Bacillus Calmette-Guérin (BCG), a live attenuated strain of Mycobacterium bovis, it is clearly the most effective and successful adjuvant immunotherapy of non-muscle invasive bladder cancer (NMIBC). In this work we summarise that training the synergy between BCG and immune checkpoint-blocking antibodies empowers macrophages to achieve their full potential in the fight against bladder cancer. We believe that this training can be further improved at different levels. On the one hand, BCG can be made more effective, for instance through genetic engineering. On the other hand, Fc-engineering of immune checkpoint-blocking antibodies may enhance the training effect of tumour cell phagocytosis by pre-activated macrophages. As a consequence, physically conjugating immune checkpoint-blocking antibodies to BCG may be an attractive option and give the bladder cancer immunotherapy script a new twist( Fig.1 and 2)
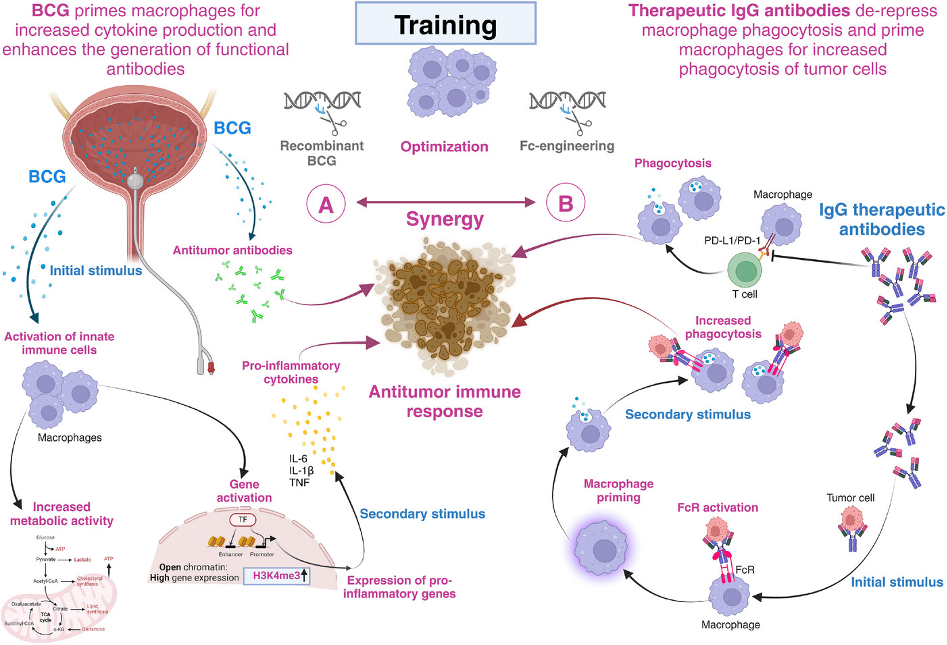
Tumour microenvironment in prostate cancer
Isabel Heidegger-Pircher, Mona Kafka, Nastasiia Artamonova, Felix Melchior
Our research group is firmly engaged in the molecular characterisation of the tumour microenvironment in prostate cancer. We recently conducted an in-depth analysis of this microenvironment for the first time using single-cell sequencing. We are currently investigating age-dependent differences in immune cell composition and tissue architecture in younger versus older patients. We are also conducting Spatial Transcriptomics in order to represent the spatial organisation and interaction of tumour and immune cells in tissue at the single-cell level. The bioinformatics analysis of these complex data is carried out in close collaboration with the Department of Bioinformatics (Z. Trajanoski).
Another project is focused on the diversity of neutrophil granulocytes in prostate cancer, a largely unexplored field. The goal is to functionally characterise different subpopulations of neutrophils in the tumour microenvironment. This project is currently being pursued as part of a doctoral thesis. We are also investigating possible disease-specific signatures and immunological peculiarities in collaboration with Internal Medicine V (D. Wolf).
We are examining the effects of intermittent fasting on tumour profiles and immune status in prostate cancer patients. Patients follow a standardised fasting protocol before planned prostatectomy or systemic therapy. Subsequently, multi-layered analyses are conducted. These include metabolomics, cytokine analysis and single-cell characterisation of peripheral immune cells. Initial results show that fasting modulates specific T-cell populations.
Active surveillance in prostate cancer
Isabel Heidegger-Pircher, Mona Kafka, Nastasiia Artamonova, Felix Melchior
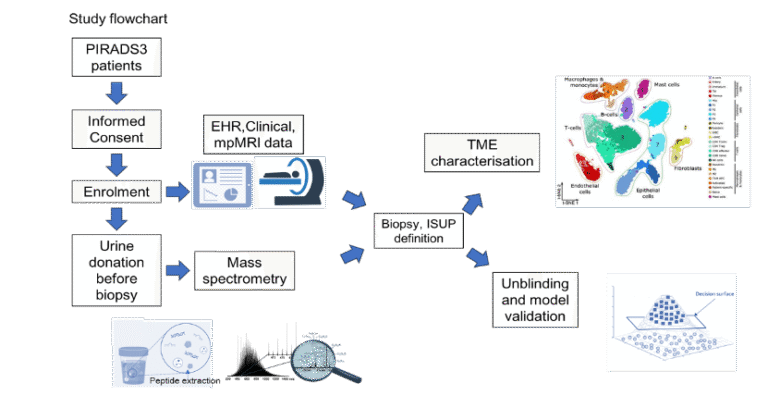
Recently, we demonstrated for the first time that changes in collagen metabolism in prostate cancer are linked to a poor prognosis. Using mass spectrometry, we diagnosed collagen-associated genes in tissue, blood and urine. These can be used as biomarkers for 1) prostate cancer (yes/no), 2) biochemical recurrence (yes/no) after prostatectomy, and 3) as prognostic markers for poor survival. In an ongoing project, we are investigating the significance of the collagen score for unclear lesions in MRI in a multicentre study (Innsbruck as the lead centre) Our aim is to spare patients unnecessary biopsies and avoid overdiagnosis and overtreatment.
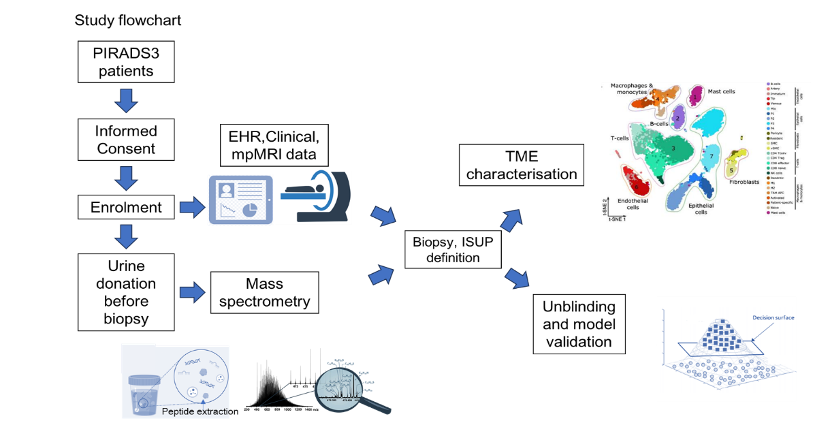
Neuroendocrine prostate cancer
Isabel Heidegger-Pircher, Mona Kafka, Nastasiia Artamonova, Felix Melchior
Neuroendocrine prostate cancer (NEPC) is a highly aggressive form of prostate cancer for which only one therapy (platinum-based chemotherapy) is currently available. Our long-term goal is to improve the care of NEPC patients. We will achieve this by understanding the molecular causes of therapy resistance and the development of NEPC, identifying new structures and concepts for therapeutic interventions, and optimising the diagnosis of emerging resistance. We are focusing on three specific areas: 1) the characterisation of the molecular mechanisms of therapy resistance in NEPC, 2) the development of blood-based biomarkers for the early detection of therapy resistance and NEPC features and 3) the development of new concepts for effective treatment of NEPC patients.
Personalised therapy of metastatic prostate cancer
Isabel Heidegger-Pircher, Mona Kafka, Nastasiia Artamonova, Felix Melchior
In the past three years, the therapeutic landscape of metastatic hormone-sensitive prostate cancer (mHSPC) has changed significantly. The introduction of combination therapies, particularly the so-called triplet therapy (androgen deprivation therapy + new hormonal therapy + docetaxel chemotherapy), has established new standards in the first-line therapy for patients with high-risk profiles. Our group was able to collect and analyse data from the world’s first multicentre real-world study on the clinical implementation of this triplet therapy in mHSPC. An update of this data analysis is currently being prepared. Our long-term research goal is to further personalise the treatment of mHSPC. Integrating molecular biomarkers is the key to improving individual risk assessment and enabling a patient-tailored therapeutic strategy.
Androgen receptor coactivators and novel targets in prostate cancer
Zoran Culig, Florian Handle, Chiara Andolfi, Martin Puhr
The research group investigated the role of a component of the MED complex, MED12. This component regulates the proliferation of androgen receptor-positive and androgen receptor-negative cancer cells. We studied how MED12 and cyclin-dependent kinase influence cellular processes in prostate cancer cells. Down-regulation of MED12 expression and CDK 8/19 activity reduced proliferation in cell lines and spheroids. We also observed a clear inhibition of the oncogene c-Myc in cancer cells. MED12 inhibition also down-regulated androgen receptor functional activity, in particular androgen receptor variant and prostate-specific antigen expression. We therefore conclude that MED12 and CDK8/19 regulate cellular processes in prostate cancer. This is a key factor in the development of malignancy and tumour progression. In collaboration with Profs. Taubert, Wach (Erlangen) and Aigner (Leipzig), we also found that miR-454-3p directly targets full-length and variant androgen receptors. The results of this project revealed new mechanisms of regulation of androgen receptor expression and activity.
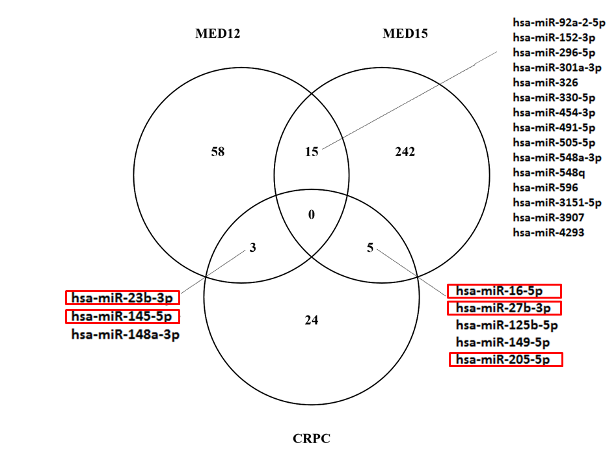
The impact of glucocorticoid administration on prostate cancer progression and therapy resistance
Martin Puhr, Florian Handle, Isabel Heidegger, Zoran Culig, Andrea Eigentler
The glucocorticoid receptor (GR) is a key molecule in the development of therapy resistance in prostate cancer. As standard androgen receptor-directed therapies fail to block the GR and GR inhibitors and might result in unspecific side effects, the identification of GR signature genes is clinically important. We investigated the altered epithelial and stromal GR signature in prostate cancer cells after glucocorticoid treatment and identified monoamine oxidase (MAO-A) as a mutual directly up-regulated epithelial and stromal GR target. MAO-A is a mitochondrial membrane-bound oxidoreductase that catalyses the degradation of biogenic and xenobiotic amines. It is up-regulated during prostate cancer progression and after glucocorticoid receptor treatment. Glucocorticoid treatment caused altered GR-target gene expression and changes in the morphology of cancer-isolated fibroblasts. Glucocorticoid treatment influences prostate cancer cell growth and the tumour microenvironment via altered GR signalling in prostate fibroblasts.
Regulation of the AR splice variant V7 by small nucleotide variants
Frédéric R. Santer, Jasper van Goubergen
The androgen receptor splice variant V7 is a hallmark of castration-resistant prostate cancer. Lacking the ligand-binding domain, this cannot be targeted by endocrine treatments for prostate cancer. Therefore, it drives the progression of the disease. AR V7 mRNA has a different 3´untranslated region. This indicates that ARV7 is subject to different regulations at the post-transcriptional level. We characterised the allelic effects of small nucleotide polymorphisms with high minor allele frequency. AR V7 is used as a therapy-guiding biomarker. These quantitative expression trait loci (eQTL) will improve the specificity of this biomarker. The project generated data showing that targeting aberrant alternative splicing at the 3´UTR of ARV7 by disturbing the CLK2/SRSF9 axis could be a valuable approach in late stages prostate cancer.
Tumour microenvironment and novel targets in prostate cancer
N. Sampson, E. Brunner, E. Damisch, L. Nommensen, M. Groeninger
The stromal tumour microenvironment (TME) is critical to the development, progression and response to the therapy of prostate cancer. Cancer-associated fibroblasts (CAFs) are the dominant cell type within the PCa TME, but display considerable heterogeneity at both the molecular and functional level. We are investigating the molecular and physical mechanisms underlying CAF plasticity and their clinical relevance on disease progression and therapy efficacy. Our aim is to identify new targets that will (i) enable the distinction between progressive vs. indolent disease and (ii) that can be exploited therapeutically in an adjuvant stromal-targeted approach. We are also investigating the role of NADPH oxidase 4 (NOX4), a ROS-producing enzyme expressed in a subpopulation of CAFs. We previously showed that this enzyme is a central mediator of a signalling hub at the tumour cell-CAF interface.
Drug repurposing in prostate cancer
Zoran Culig, Henrike Held, Martin Puhr
The development of new drugs for prostate cancer is expensive. A second strategy is based on computational analysis of drugs currently used for other diseases. A new project has been initiated. Its aim is to identify drugs that can target therapy-resistant prostate cancer.
Pictures
Selected Publications
Pichler R, Siska PJ, Tymoszuk P, Martowicz A, Untergasser G, Mayr R, Weber F, Seeber A, Kocher F, Barth DA, Pichler M, Thurnher M (2023): “A chemokine network of T cell exhaustion and metabolic reprogramming in renal cell carcinoma.” Front Immunol 14:1095195.
Klaver D, Thurnher M (2023): “P2Y11/IL-1 receptor crosstalk controls macrophage inflammation: a novel target for anti-inflammatory strategies?” Purinergic Signal. 19(3):501.
Pichler R, Diem G, Hackl H, Koutník J, Mertens LS, D Andrea D, Pradere B, Soria F, Mari A, Laukhtina E, Krajewski W, Teoh JY, Del Guidice F, Moschini M, Thurnher M, Posch W (2023): “Intravesical BCG in bladder cancer induces innate immune responses against SARS-CoV-2.” Front Immunol. 14:1202157.
Andrea Katharina Lindner, Agnieszka Martowicz, Gerold Untergasser, Johannes Haybaeck, Eva Compérat, Florian Kocher, Andreas Seeber, Martin Thurnher, Renate Pichler (2023): “CXCR3 Expression is Associated with Advanced Tumor Stage and Grade Influencing Survival after Surgery of Localised Renal Cell Carcinoma.” Cancers (Basel). 15(4):1001.
Klaver D, Gander H, Frena B, Amato M, Thurnher M (2024): “Crosstalk between purinergic receptor P2Y11 and chemokine receptor CXCR7 is regulated by CXCR4 in human macrophages.” Cell Mol Life Sci. 81(1):132.
Pichler R, Stäblein J, Mari A, Afferi L, D’Andrea D, Marcq G, Del Giudice F, Soria F, Caño-Velasco J, Subiela JD, Gallioli A, Tully KH, Mori K, Herms A, Pradere B, Moschini M, Mertens LS, Thurnher M, European Association of Urology-Young Academic Urologists (EAU-YAU): Urothe
Pichler R, Diem G, Hackl H, Koutník J, Mertens LS, D Andrea D, Pradere B, Soria F, Mari A, Laukhtina E, Krajewski W, Teoh JY, Del Guidice F, Moschini M, Thurnher M, Posch W. Intravesical BCG in bladder cancer induces innate immune responses against SARS-CoV-2. Front Immunol. 2023 Jul 13;14:1202157. doi: 10.3389/fimmu.2023.1202157. PMID: 37520557; PMCID: PMC10374029.
Lindner AK, Lackner F, Tymoszuk P, Barth DA, Seeber A, Kocher F, Toth B, Hochleitner M, Pichler M, Pichler R. Sex hormones influence survival of patients with metastatic urothelial carcinoma undergoing immune checkpoint therapy. Biol Sex Differ. 2023 Jun 5;14(1):38. doi: 10.1186/s13293-023-00522-x. PMID: 37277835; PMCID: PMC10243034.
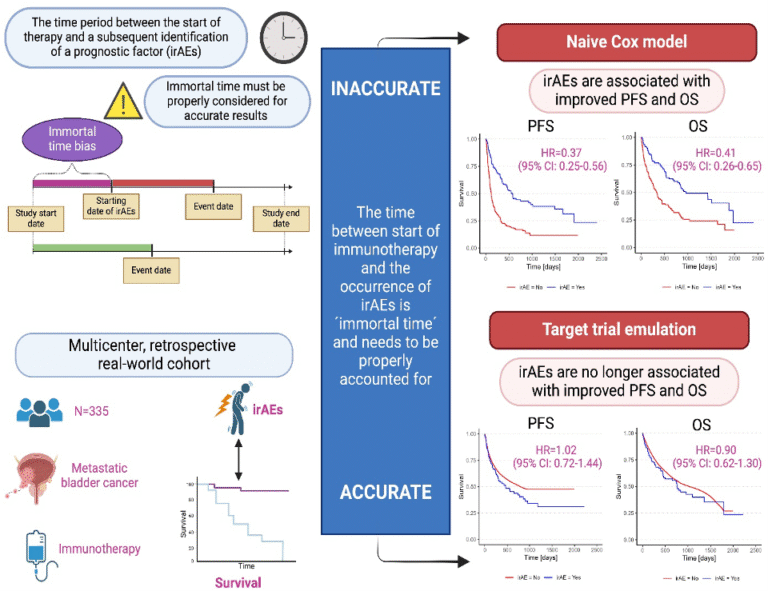
Pichler R, Fritz J, Maier S, Hassler MR, Krauter J, D Andrea D, Laukhtina E, Gust K, Mori K, Tully KH, Niedersuess-Beke D, Korber L, Spiegelberg JA, Bauernhofer T, Subiela JD, Mayr R, Kronbichler A, Moschini M, Teoh J, Pradere B, Shariat SF, Ulmer H, Mertens LS; European Association of Urology–Young Academic Urologists (EAU-YAU): Urothelial Carcinoma Working Group. Target trial emulation to evaluate the effect of immune-related adverse events on outcomes in metastatic urothelial cancer. Cancer Immunol Immunother. 2024 Dec 21;74(1):30. doi: 10.1007/s00262-024-03871-7. PMID: 39708183; PMCID: PMC11663210.
Pichler R, Fritz J, Mari A, Cadenar A, von Deimling M, Marcq G, Del Giudice F, Leonardo C, Bologna E, Mori K, Tahbaz R, De Santis M, Klatte T, Erber B, Lackner F, Kronbichler A, Seeber A, Fisch M, Moschini M, Pradere B, Mertens LS. Cisplatin eligibility in the neoadjuvant setting of patients with muscle-invasive bladder cancer undergoing radical cystectomy. Oncologist. 2024 Nov 4;29(11):e1511-e1522. doi: 10.1093/oncolo/oyae160. PMID: 38956801; PMCID: PMC11546640.
Pichler R, Thurnher M. Training the synergy between Bacillus Calmette-Guérin and immune checkpoint-blocking antibodies in bladder cancer. Cancer Commun (Lond). 2025 Jan 10. doi: 10.1002/cac2.12647. Epub ahead of print. PMID: 39797503.
Schoepf AM, Gebhart M, Federspiel M, Heidegger I, Puhr M, Hotze M, et al.
Eradication of Therapy-Resistant Cancer Stem Cells by Novel Telmisartan Derivatives.
J Med Chem. 2025 Jan 9;68(1):287-306. doi: 10.1021/acs.jmedchem.4c01865. PMID: 39693499. [Free PMC article]
Scheiber A, Trebo M, Pittl A, Heidegger I, Hautz T, Oberhuber R, et al.
Profiling low-mRNA content cells in complex human tissues using BD Rhapsody single-cell analysis.
STAR Protoc. 2024 Dec 20;5(4):103475. doi: 10.1016/j.xpro.2024.103475. PMID: 39661509. [Free PMC article]
Giannini G, Kafka M, Neuwirt H, Artamonova N, Santo GD, Virgolini I, et al. (incl. Heidegger I).
Safety and Efficacy of 177Lu-PSMA Therapy Following 223Radium Treatment: A Retrospective Multinational Real-World Analysis.
Clin Genitourin Cancer. 2025 Feb;23(1):102260. doi: 10.1016/j.clgc.2024.102260. PMID: 39608079.
Frantzi M, Morillo AC, Lendinez G, Blanca A, Lopez Ruiz D, Parada J, et al. (incl. Heidegger I).
Validation of a Urine-Based Proteomics Test to Predict Clinically Significant Prostate Cancer: Complementing mpMRI Pathway.
Pathobiology. 2024 Nov 11:1-10. doi: 10.1159/000542465. PMID: 39527943.
Steinkohl F, Luger AK, Gruber L, Pichler R, Heidegger I, Bektic J, Aigner F.
Patients’ anxieties and fears: a comparison between transrectal prostate biopsy and prostate MRI.
Transl Androl Urol. 2024 Oct 31;13(10):2201-2208. doi: 10.21037/tau-24-239. PMID: 39507871. [Free PMC article]
Artamonova N, Kafka M, Faiss L, Avetisyan D, Puche Sanz I, La Bombarda G, Iacono G, Zattoni F, Steiner E, D’Elia C, Pycha A, Ladurner M, Jagodic S, Gandaglia G, Heidegger I.
Impact of Renin-Angiotensin System Inhibitors on Disease Characteristics in Patients with Localized Prostate Cancer Treated with Radical Prostatectomy: A European Association of Urology Young Academic Urologists Prostate Cancer Working Group Multi-institutional Study.
Andolfi C, Bartolini C, Morales E, Gündogdu B, Puhr M, Guzman J et al. MED12 and CDK 8/19 modulate androgen receptor activity and enzalutamide response in prostate cancer. Endocrinology 165:bqae114, 2024.
Guzman J, Hart M, Weigelt K, Neumann A, Aigner A, Andolfi C et al. The microRNA miR-454 and the mediator complex component MED12 are regulators of the androgen receptor pathway in prostate cancer. Sci Rep 15:10272, 2025.
Culig Z, Puhr M. Androgen receptor-interacting proteins in prostate cancer development and therapy resistance. Am J Pathol 194:324, 2024.
Eigentler A, Handle F, Schanung S, Degen A, Hackl H, Erb H et al. “Glucocorticoid treatment influences prostate cancer cell growth and the tumor microenvironment via altered glucocorticoid receptor signalling in prostate fibroblasts” Oncogene 43:235, 2024.
Van Goubergen J, Perina M, Handle F, Morales E, Kremer A, Schmidt O et al. “Targeting the CLK2/SRSF9 splicing axis in prostate cancer leads to decreased ARV7 expression”, Mol Oncol 19:496, 2025.
Selection of Funding
Felix Melchior – Richard Übelhör grant ÖGU (10.000 Euro)
Isabel Heidegger-HORIZON-MSCA-2023-DN-01 (total project volume 2.049.184 Euro → Innsbruck 270.312 Euro, Co-PI Z. Culig)
Isabel Heidegger-Hans und Herta Tuba Stiftung Research Grant (100.000 Euro)
Isabel Heidegger- AstraZeneca Independent Research Grant (12.200 Euro)
DFG FWF Project to Zoran Culig “microRNA and regulation of MED in prostate cancer”, project completed.
Austrian National Bank project to Martin Puhr „The Impact of Glucocorticoid Administration on Prostate Cancer Progression and Therapy Resistance“, project completed.
The Fondation Cancer Luxembourg grant to Frédéric R. Santer “Androgen receptor splice variant AR-V7 in advanced prostate cancer. Improvement of biomarker specificity”, project completed.
FWF SNF project to Natalie Sampson “Therapy resistance in prostate cancer: role of tumor stroma heterogeneity”
FWF project to Natalie Sampson “Functional significance of NADPH oxidaxe 4 in the prostate cancer stromal tumor microenvironment”
EU project PROMOTE “Prostate cancer omics oriented intervention” (Industrial doctorate programme) to Zoran Culig.
Collaborations
Renate Pichler:
Prof. Michael M. Shen, Columbia University Irving Medical Center, Herbert Irving Comprehensive Cancer Center, New York, US
Prof. Roger Li, Moffitt Cancer Center, Tampa, FL, US
Dr. Henning Plage, Department of Urology, Charité Campus Benjamin Franklin, Berlin, Germany
Dr. Florestan Koll, Department of Urology, Medical University of Graz, Austria
Bernhard Englinger PhD, Department of Urology, Medical University Vienna, Austria
Prof. Mieke Van Hemelrijck, Comprehensive Cancer Center, Guy`s Hospital, London, UK