Anichstraße 35
6020 Innsbruck
Fax: +43 (0)50 504 22824
Email: Rohit.Arora@i-med.ac.at
Website: https://ortho-trauma-innsbruck.tirol-kliniken.at/
Research year
Research Branch (ÖSTAT Classification)
102001, 104026, 106013, 106022, 106024, 106039, 106049, 206001, 211904, 301103, 301105, 301107, 301114, 301304, 301305, 301902, 301904, 302019, 302029, 302037, 302044, 302048, 302053, 302055, 302057, 302063, 302070, 302085, 303002,303020, 303021, 303025, 303041, 304002, 305103, 305104, 305106, 305109, 305901, 305905, 305908, 601026
Keywords
arthroplasty, biofilm genetics, biomechanics, Cell biology, Clinical studies, fracture fixation, implant-related infections, microstructural bone failure, minimal-invasive surgery, and orthopaedics
Research Focus
Our research focuses on the evaluation, development and improvement of new and existing treatments for traumatic and degenerative musculoskeletal diseases. We are undertaking clinical randomised studies; biomechanical cadaver tests to improve implant behaviour; morphologic/cell biological investigations on ageing processes; and biofilm investigations on implant-related infections. 3D reconstruction, gait lab and micro CT allow research at the highest level.
General Facts
The Department is organized into clinical teams specializing in anatomical regions. A networked infrastructure provides our clinicians with a wide range of research. The setting supports clinical and laboratory research at macroscopic, microscopic and cellular level. Clinical research features retrospective and prospective studies, medical device studies and trials of treatment methods and their success supported by our study coordination team. Our gait lab deals with movement analysis and optimization and material testing machines are available for the in vitro testing of soft and hard biological tissues in the biomechanics lab, with several custom-made test setups for various anatomical regions and joint simulators for the spine, knee, shoulder and wrist. Biomedical engineering work aims at improving techniques for filling bony defects. The morphology and cell biology investigations are based on the intervertebral disc cell (IVD), especially on traumatized IVDs and degenerated IVDs from patients with a degenerative disc disease. Osteoporosis and its mechanisms are studied in a fully equipped tissue culture unit. The biofilm lab concentrates on implant-related infections and aims to understand biofilm building and attachment to biomaterial surfaces. The two micro-CT scanners are used for image reconstruction, quantitative analysis and 3D visualization.
Research
Clinical Research – Study Coordination
Mariëtte Widner, MSc
We provide support for the organization of all types of clinical trials, ensuring compliance with local and international regulations and approvals including financial and contractual aspects. Our research focuses on the improvement of surgical and postoperative treatments and on developing innovative devices and methods. Our goal is to achieve long-term quality assurance and evidence-based medicine at the highest level. Our studies include fracture fixation and reduction, prevention of implant failure and infection, spinal cord injury improvement, joint arthroplasty, knee arthroscopy (cartilage repair), sports-related injuries, return to sports and basic clinical research on factors that influence outcomes.
The Study Coordination acts as an for a wide range of projects, enabling work with hyperspectral imaging and new molecular biology sequencing techniques to detect bone infections in clinical settings. An interdisciplinary project with the Institute of Hygiene and Medical Microbiology has investigated the efficacy of the body’s own antiseptic N-chlortaurine (NCT), which showed superior bactericidal activity to the antibiotic vancomycin. The results will be published in 2025.
Another focus is on hip fracture prophylaxis, which we are investigating in a trial with pharmaceutical sponsor AgNovos Healthcare. The study is examining calcium-based bone cement (AGN1 LOEP) applied to the unfractured contralateral hip, which hardens intraoperatively and turns into bone after 5-7 years to reduce the risk of fracture after falls. We are also involved in a clinical trial on endoscopic carpal tunnel release (ECTR) using a new modified paediatric urethrotome with retracted scalpel (Tarfusser et al; Wolf GmbH), designed to overcome known risks and to minimize trauma while providing the best possible view.
Biomechanics Laboratory
Werner Schmölz, Univ. Prof., PhD, Dipl.-Ing (FH)
Implant anchorage in stabilization procedures for patients with reduced bone quality remains a challenge. New test setups, loading protocols and algorithms have been developed to detect implant loosening during dynamic loading. We used the protocols to investigate implant anchorage in various anatomical regions with different augmentation techniques, materials and surgical methods.
For spinal pathologies, we examined various instrumentations and surgical techniques for spine biomechanics. Cement augmentation of pedicle screws is a well established procedure to improve screw anchorage in reduced bone quality but it increases the risk of complications. We have shown that augmenting only the four marginal screws in long instrumentations provides sufficient anchorage while minimizing the risks of PMMA augmentation. We have also confirmed that lateral misplacement of pedicle screws negatively impacts anchorage, while redirection of misplaced screws does not improve anchorage and could further compromize it, even if it does not harm anatomical structures.
An interdisciplinary project with the Institute of Hygiene and Medical Microbiology, funded by the TWF, has investigated the effects of various antiseptic treatments on ligament grafts. We found no significant differences in ultimate load, elongation, stiffness or elastic modulus between native untreated specimens and those treated with N-chlortaurine (NCT) or vancomycin.
In knee ligament injuries, lateral extra-articular tenodesis (LET) as an adjunct to anterior cruciate ligament reconstruction (ACLR) reduces ACLR graft forces under internal tibial torque and decreases residual rotational laxity after ACLR, potentially reducing the risk of re-ruptures. We have also compared the Larson and Arciero techniques for posterolateral knee ligament reconstructions and found that the Arciero technique provided greater stability in tibial external rotation at higher flexion angles.
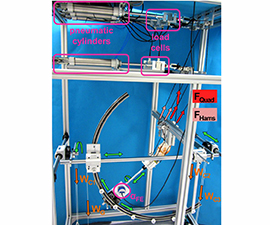
Orthopaedic Gait lab
Dipl. Ing. Stefan Fischler
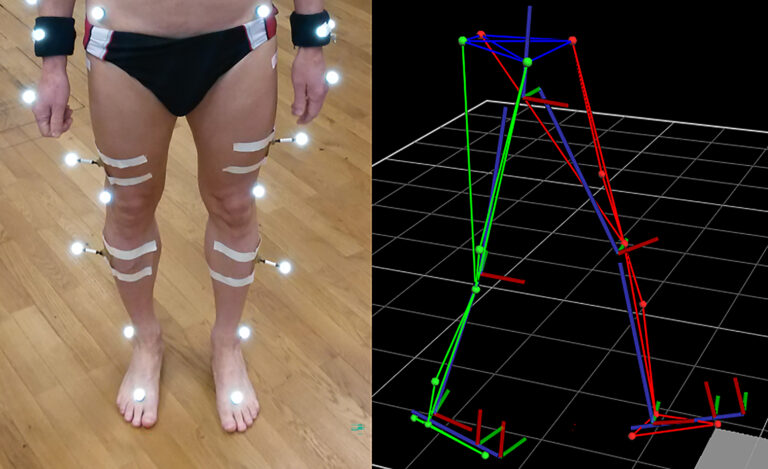
Research in the biomechanical gait lab focuses on movement analysis and optimization, with the instruments of the gait lab used to detect unhealthy loading or to reveal differences in gait speed. We use a Vicon system to analyse everyday movements and collect information to improve ergonomic behaviour. The system consists of a Vicon Sync-Box (lock lab) with eight infrared cameras, two colour cameras 720p and two pressure plates, with Vicon Nexus 2 serving as recording and evaluation software (figure 2). In September 2024, prophysics AG adapted the system to solve technical problems of the Vicon Giganet, replacing it for a Vicon Syn-Box lock lab.
Current studies are focusing on movement behaviour, for example before and after knee surgery (arthroplasty, trochleoplasty, cruciate ligament fixation). By determining all possible changes in the range of motion and ground reaction forces, we can investigate the best postoperative outcome of various treatment methods and implants.
We are also determining the change in patient mobility after scoliosis operations. Again, the range of motion (total angle of rotation between hip and torso) is of special interest before and after surgery.
Morphology and Cell Biology – intervertebral disc investigations
PD Dr. med. Ingrid Sitte, Miranda Klosterhuber BMA
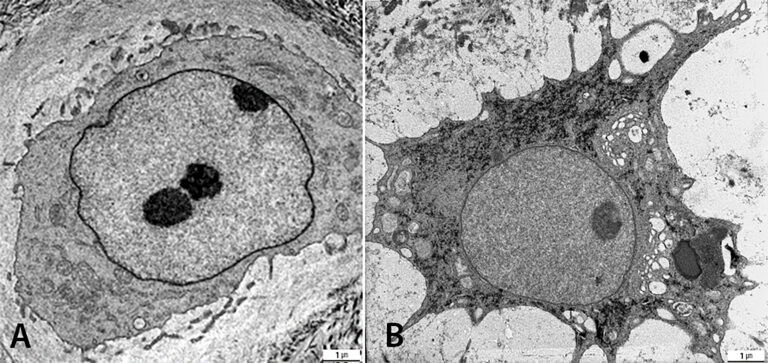
We are studying degenerated and traumatized IVDs of the cervical and lumbar spine by light microscopy and by transmission electron microscopy (TEM). The group is investigating two parameters of traumatized intervertebral discs of the cervical spine: fracture mechanism (compression and rotation fractures) and degeneration grade (low and high). Disc architecture is studied histologically and cell morphology examined ultra-structurally to quantify cell death and healthy cells. Investigations of the degenerative disc diseases revealed a new disc cell morphology, the balloon cell, which we first detected by transmission electron microscopy. We are now examining these special cell in detail. They seem to be part of the ageing processes in the IVD, cartilage and adjacent bone structures, which can be studied with our SPARC KO Mouse experiment. The group has also investigated intervertebral disc cells of the degenerated cervical und lumbar spine with prolapse/protrusion and/or osteochondrosis. Whereas balloon cells were obvious in the disc matrix, with a mean of 40% in prolapsed cervical discs, cell culture featured up to 98% balloon cells in the degenerated herniated disc in human samples. This special cell morphology has diminished SPARC (Osteonectin), especially in the nucleus of human cells and in cells from SPARC Null KO mice (figure 3). An analysis of pro-inflammatory and pro-apoptotic proteins of human disc cells might provide further insights and more information on the consequences. The findings may have an impact on future therapy.
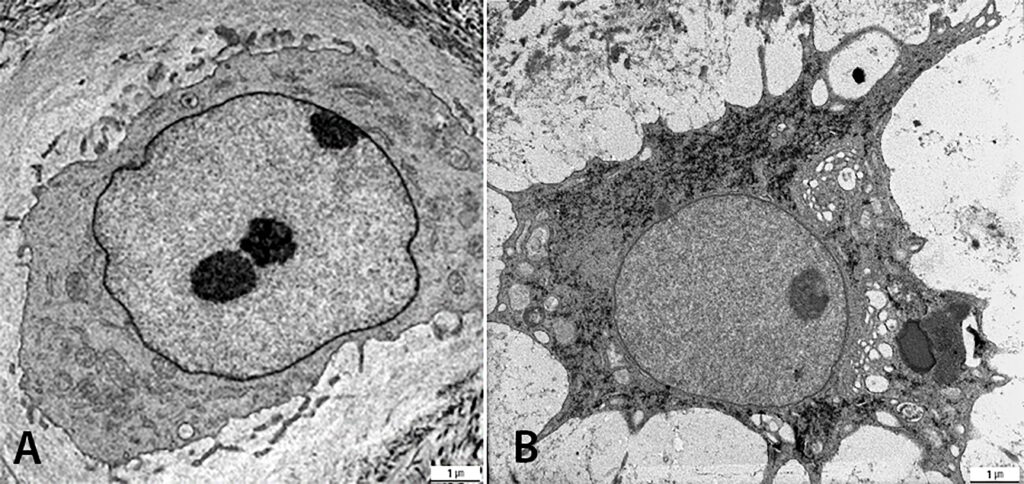
Biofilm Lab – implant-related infections
Ass. Prof. Dr. Deborah Coraça-Huber, PhD, MMSc; Dr. Christopher Spiegel, PhD
An improved understanding of the structure and function of biofilms (figure 4) will help us develop treatments to avoid infections, to develop new diagnostic tools and to treat infections.

Biofilms-specific genes and the diagnosis of PJIs
A bacterial biofilm is produced in a two-step process: initial bacterial attachment to a surface followed by a biofilm formation, consisting of bacterial proliferation, intercellular adhesion and extracellular production of slime substance. Different genes are involved in each phase of the biofilm formation.
Osseo integration of titanium implants aiming biofilm prevention and peri-implantitis prevention.
We have performed an interdisciplinary project involving dental medicine and orthopaedics, aiming at the development of a titanium surface able to avoid the attachment of bacteria and biofilm formation. The results will be translated to the orthopaedic area.
Natural antimicrobial substances
Alternative substances to antibiotics can be administered alone, locally, for the treatment of wound infections and implant infections. Such substances can also act as coadjutants, increasing the activity of systemically applied antimicrobials.
Metagenomics sequencing
The MG Oxford Nanopore Sequencing tool enables us to detect microbial population in a range of substrates, including human fluids such as joint aspirates, biopsies and sonication fluids. Together with the ability to detect antibiotic resistance genes, the new technology can help develop new diagnostic tools for PJI.
Biofilm-related oncogenesis
If they come into contact with human tissue, biofilms can lead to oncogenesis. Biofilm-related oncogenesis can develop in several tissues and systems. The most frequently studied is the oncogenesis of colon carcinoma after the transition of a healthy microbiome in a pathogenic form of biofilm. The knowledge can help us understand bone tissue cancer and its probable relation to a prior infection and biofilm formation.
Reconstruction techniques and implant fixation
PD David Putzer, PhD, MSc
We are using bone transplant bone chips to reconstruct and fill bone defects, which often have multifactorial causes. They may arise after tumour removal, cranio-maxillofacial surgery or total hip revisions. Bone grafting involves filling defects with autogenous, allogeneic or artificial materials. Furthermore, our research gains necessary insights to impaction forces and their effects on the human body.
It is crucial to analyse the minimum force needed for safe acetabular cup impaction to avoid fractures in hip surgeries. Similar considerations apply to knee and shoulder surgeries, though most impaction processes are not standardized.
Implant migration follow-up X-rays can reveal implant migration using the EBRA technique, which helps predict early implant failure and assess design effects of various systems. Bone cement is crucial for fixing artificial joints. We are studying reinforcement methods using wires or additives such as carbon. In addition to fixation, bone cement can also deliver antibiotics to treat implant-related infections.
Core facility – Micro CT/3D reconstruction/ Multimodal imaging
Ass.-Prof. PD MMag. Dr. rer. nat. Pallua Johannes, MSc PhD
Micro-CT in musculoskeletal research and in the clinic
Micro-computed tomography (micro-CT) is a high-resolution X-ray imaging technique that enables the non-destructive evaluation of biological tissues and biomaterials, such as assessing bone defect repair or material microstructures in implants. Recent studies have emphasized its role in diagnosing bone infections and assessing mechanical stability influenced by antibiotic-loaded bone cement.
The Core Facility Micro-CT has two state-of-the-art systems: Scanco vivaCT 40 and XtremeCT II. Supported by high-performance workstations (e.g. quantitative morphometry), the systems enable the precise quantification of structural and density parameters and the creation of detailed 3D models for research and clinical applications.
Application of 3D models in orthopaedic research and surgery
Advances in imaging and modeling software now allow the creation of accurate, patient-specific 3D models from CT, MRI or 3D ultrasound data. The models support various clinical and research applications, including:
• Structural analysis and virtual planning of surgery
• 3D printing of anatomical models for implant fitting and surgical rehearsal
• Design of patient-specific surgical guides and templates
Innovative research directions and technologies
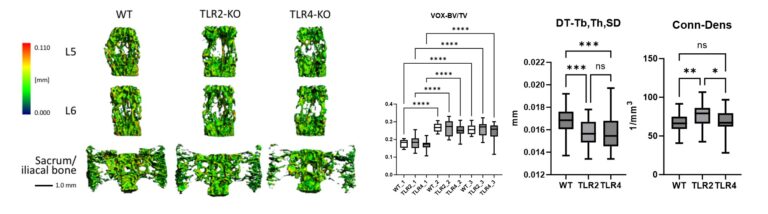
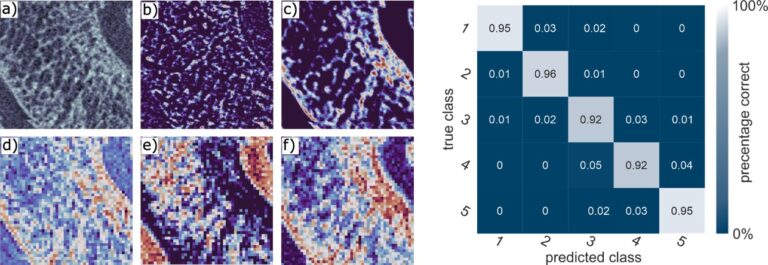
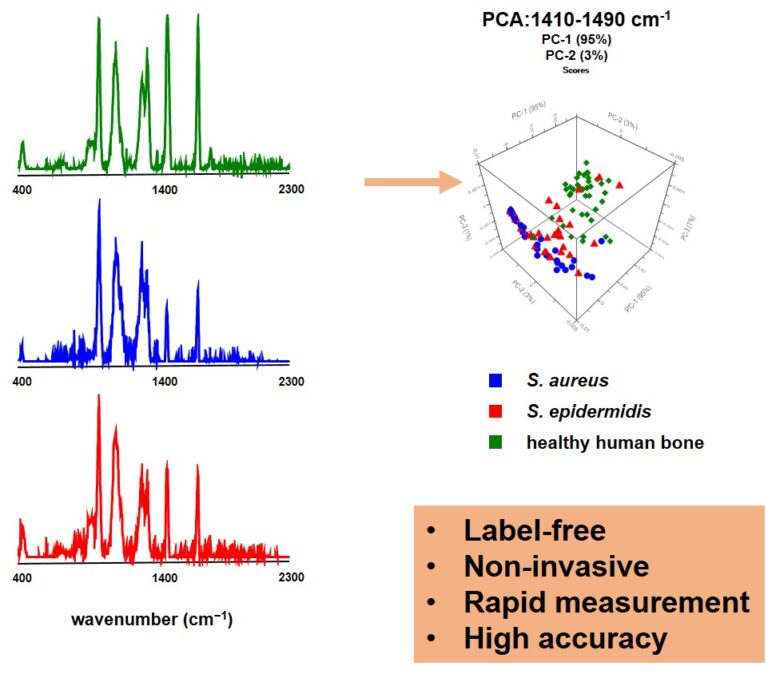
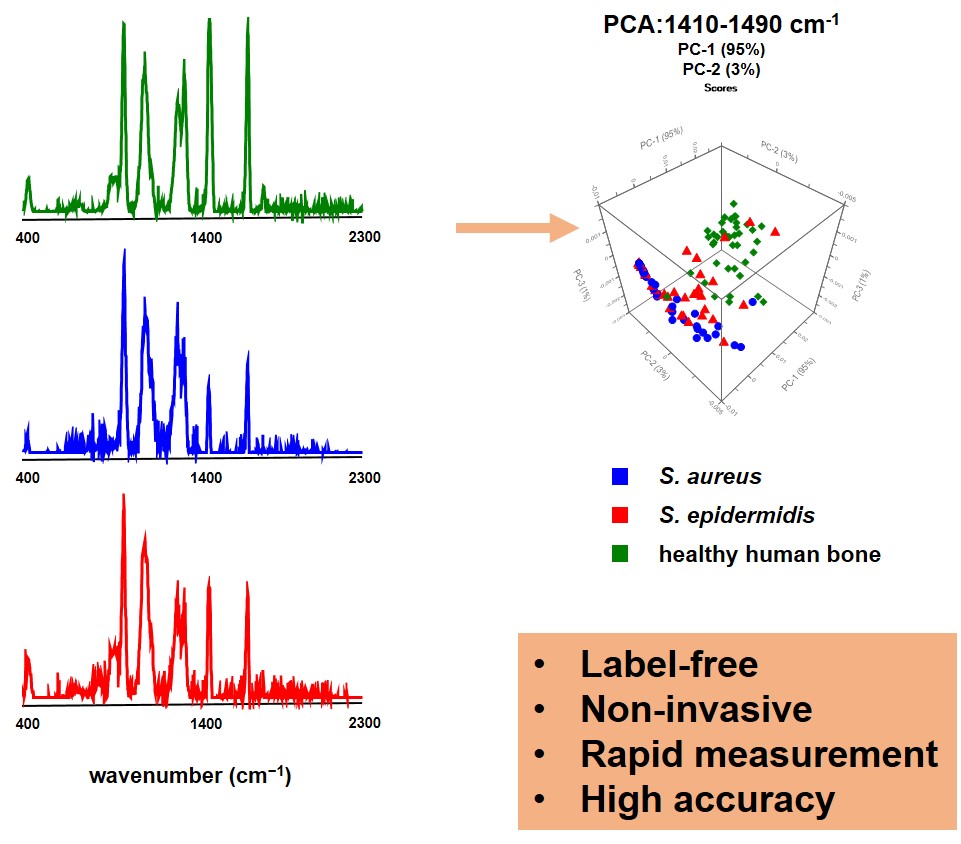
Figure 5 shows high-resolution micro-CT scans of trabecular bone. Quantitative metrics such as bone volume fraction (VOX-BV/TV), trabecular thickness and connectivity density reveal the impact of Toll-like receptors on bone structure. We are also applying AI-driven convolutional neural networks to automate motion artefact grading in high-resolution CT datasets, improving diagnostic precision and efficiency. We are also evaluating Raman handheld spectroscopy for real-time pathogen identification in bone infections during surgery, aiding surgical decision-making.
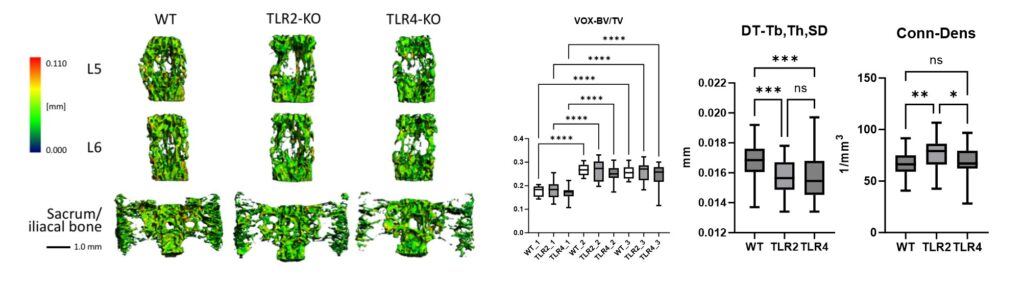
Representative micro-CT scans of the sacrum and lumbar vertebrae (L5, L6) from wildtype (WT), TLR2-knockout (TLR2-KO) and TLR4-knockout (TLR4-KO) mice. Quantitative analysis highlights differences in bone volume fraction (VOX-BV/TV), trabecular thickness (DT-Tb,Th,SD) and connectivity density (Conn-Dens), showing the impact of Toll-like receptors on bone structure.
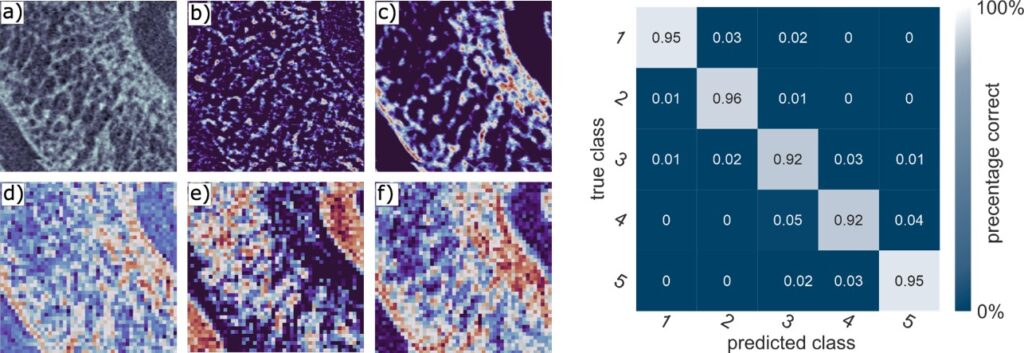
Assessment of motion artefacts using AI-driven deep convolutional neural networks (3D-CNN), comparing manual observer ratings with AI-based motion correction. The figure includes a confusion matrix illustrating the classification performance across different image quality categories.
Pictures
Selected Publications
Clinical Studies
Benedikt, S.; Horling, L.; Stock, K.; Degenhart, G.; Pallua, J.; Schmidle, G.; Arora, R.: The impact of motion induced artifacts in the evaluation of HR-pQCT scans of the scaphoid bone: an assessment of inter- and intraobserver variability and quantitative parameters.
QUANTITATIVE IMAGING IN MEDICINE AND SURGERY. 2023; 13(3); 1336.
PubMed: 36915364 doi: 10.21037/qims-22-345 [FLDID: 140358] IF: 2,900 / ZIT: 0
Benedikt, Stefan; Rieser, Lukas; Schmidle, Gernot; Stock, Kerstin; Horling, Lukas; Degenhart, Gerald; Arora, Rohit: Influence of demographic factors on the occurrence of motion artefacts in HR-pQCT.
ARCHIVES OF OSTEOPOROSIS. 2023; 18(1); 142.
PubMed: 38008822 doi: 10.1007/s11657-023-01352-5 [FLDID: 140649] IF: 3,000 / ZIT: 0
Dankl, Lukas; Crepaz-Eger, Ulrich; Arora, Rohit; Schneider, Friedemann: Retrospective Analysis of Nosocomial SARS-CoV-2 Infections in Orthopedic and Traumatological Inpatients.
HEALTHCARE. 2023; 11(20); 2765.
PubMed: 37893839 doi: 10.3390/healthcare11202765 [FLDID: 140795] IF: 2,800 / ZIT: 0
Eichinger, Martin; Ploner, Martin; Degenhart, Gerald; Rudisch, Ansgar; Smekal, Vinzenz; Attal, Rene; Mayr, Raul: Tunnel widening after ACL reconstruction with different fixation techniques: aperture fixation with biodegradable interference screws versus all-inside technique with suspensory cortical buttons. 5-year data from a prospective randomized trial.
ARCHIVES OF ORTHOPAEDIC AND TRAUMA SURGERY. 2023; 143(11); 6707-6718.
PubMed: 37542556 doi: 10.1007/s00402-023-05001-x [FLDID: 139637] IF: 2,300 / ZIT: 0
Hoellerer, Dominik; Kaiser, Peter; Runer, Armin; Steiner, Ekkehard; Koidl, Christian; Arora, Rohit; Schneider, Friedemann: Injury Incidence, Outcomes, and Return to Competition Times after Sports-Related Concussions during One Professional Ice Hockey Season: A Prospective Cohort Study.
HEALTHCARE. 2023; 11(24); 3153.
PubMed: 38132042 doi: 10.3390/healthcare11243153 [FLDID: 140687] IF: 2,800 / ZIT: 0
Biomechanic Lab
Spicher A, Lindtner RA, Zegg MJ, Schmid R, Hoermann R, Schmoelz W. Pedicle screw augmentation in posterior constructs of the thoracolumbar spine: How many pedicle screws should be augmented? Clin Biomech (Bristol). 2023 Jun;106:106010. doi: 10.1016/j.clinbiomech.2023.106010. Epub 2023 May 24. PMID: 37245280.
Sigloch M, Mayr R, Glodny B, Coppola C, Hoermann R, Schmoelz W. Modified Lemaire Tenodesis Forces in Cadaveric Specimens Are Not Affected by Random Small-Scale Variations in the Femoral Insertion Point During Active Knee Joint Flexion-Extension. Arthrosc Sports Med Rehabil. 2023 May 31;5(3):e799-e807. doi: 10.1016/j.asmr.2023.04.007. PMID: 37388897; PMCID: PMC10300583.
Sigloch M, Coppola C, Hoermann R, Alt P, Schmoelz W, Mayr R. Overconstraint Associated With a Modified Lemaire Lateral Extra-Articular Tenodesis Is Decreased by Using an Anterior Femoral Insertion Point in a Cadaveric Model. Arthroscopy. 2024 Aug 22:S0749-8063(24)00573-5. doi: 10.1016/j.arthro.2024.07.041. Epub ahead of print. PMID: 39173687.
Coppola C, Sigloch M, Hoermann R, Schlumberger M, Schuster P, Schmoelz W, Mayr R. Posterolateral Knee Ligament Reconstruction Using the Arciero Technique Provides Greater Rotational Stability Than the Modified Larson Technique: A Biomechanical Study. Am J Sports Med. 2025 Jan;53(1):147-153. doi: 10.1177/03635465241294072. PMID: 39741489; PMCID: PMC11689966.
Schmoelz W, Spicher A, Lindtner R, Hörmann R, Srour R. In vitro biomechanical evaluation of a strutted intradiscal spacer for lumbar discectomy. Clin Biomech (Bristol). 2025 Mar 12;124:106491. doi: 10.1016/j.clinbiomech.2025.106491. Epub ahead of print. PMID: 40121997.
microCT/3D Reconstruction:
S.Benedikt, Kerstin Stock, Lukas Horling,G. Schmidle, M. Schirmer, G. Degenhart, M. Blauth, C. Lamina, J. D. Pallua and R. Arora: Bone remodelling after scaphoid fractures: HR-pQCT, clinical and laboratory data from a prospective 1-year follow-up study. Bone (2025): https://doi.org/10.1016/j.bone.2024.117337
J. D. Pallua, C. Louis, N. Gattermair, A. Brunner, B. Zelger, M. Schirmer, J. Badzoka, C. Kappacher, C. W. Huck, J. Popp, W. Rabl and C. Wöss: Raman Handheld Versus Microscopic Spectroscopy for Estimating the Post-Mortem Interval of Human Bones: A Comparative Pilot Study. Bioengineering (2024): https://doi.org/10.3390/bioengineering11111151
S. Benedikt, P. Zelger, L. Horling, K. Stock, J. D. Pallua#, M. Schirmer, G. Degenhart, A. Ruzicka, and R. Arora: Deep Convolutional Neural Networks Provide Motion Grading for High-Resolution Peripheral Quantitative Computed Tomography of the Scaphoid. Diagnostics (2024):https://doi.org/10.3390/diagnostics14050568 #Correspondence
J. D. Pallua#, A. K. Pallua, W. Streif ,H. Spiegl, C. Halder, R. Arora and M. Schirmer: Long-Term Comparison of Two- and Three-Dimensional Computed Tomography Analyses. of Cranial Bone Defects in Severe Parietal Thinning. Diagnostics (2024): https://doi.org/10.3390/diagnostics14040446 #Correspondence
V.M. Schmidt, P. Zelger, C. Wöss, M. Fodor, T. Hautz, S. Schneeberger, C. W. Huck, R. Arora, A. Brunner, B. Zelger, M. Schirmer, J. D. Pallua#: Handheld hyperspectral imaging as a tool for the post-mortem interval estimation of human skeletal remains. Heliyon (2024): https://doi.org/10.1016/j.heliyon.2024.e25844 #Correspondence
Biofilm Lab
Spiegel C, Ünalan B, Kaserbacher A, Arora R, Coraça-Huber DC. Eicosapentaenoic Acid and Docosahexaenoic Acid as an Antimicrobial Agent in Orthopedics-An In Vitro Study About the Race for Surface. Pathogens. 2025 Jan 10;14(1):57. doi: 10.3390/pathogens14010057. PMID: 39861018; PMCID: PMC11768219.
Spiegel C, Coraça-Huber DC, Nogler M, Arora R, Putzer D. Cold Plasma Activity Against Biofilm Formation of Prosthetic Joint Infection Pathogens. Pathogens. 2024 Dec 28;14(1):10. doi: 10.3390/pathogens14010010. PMID: 39860971; PMCID: PMC11768226.
Hickok NJ, Li B, Oral E, Zaat SAJ, Armbruster DA, Atkins GJ, Chen AF, Coraça-Huber DC, Dai T, Greenfield EM, Kasinath R, Libera M, Marques CNH, Moriarty TF, Scott Phillips K, Raghuraman K, Ren D, Rimondini L, Saeed K, Schaer TP, Schwarz EM, Spiegel C, Stoodley P, Truong VK, Tsang SJ, Wildemann B, Zelmer AR, Zinkernagel AS. The 2023 Orthopedic Research Society’s international consensus meeting on musculoskeletal infection: Summary from the in vitro section. J Orthop Res. 2024 Mar;42(3):512-517. doi: 10.1002/jor.25774. Epub 2024 Jan 12. PMID: 38146070.
Blanco Massani M, To D, Meile S, Schmelcher M, Gintsburg D, Coraça-Huber DC, Seybold A, Loessner M, Bernkop-Schnürch A. Enzyme-responsive nanoparticles: enhancing the ability of endolysins to eradicate Staphylococcus aureus biofilm. J Mater Chem B. 2024 Sep 25;12(37):9199-9205. doi: 10.1039/d4tb01122h. PMID: 39263769.
Schwarz EM, Archer NK, Atkins GJ, Bentley KLM, Botros M, Cassat JE, Chisari E, Coraça-Huber DC, Daiss JL, Gill SR, Goodman SB, Harro J, Hernandez CJ, Ivashkiv LB, Kates SL, Marques CNH, Masters EA, Muthukrishnan G, Owen JR, Raafat D, Saito M, Veis DJ, Xie C. The 2023 Orthopaedic Research Society’s International Consensus Meeting on musculoskeletal infection: Summary from the host immunity section. J Orthop Res. 2024 Mar;42(3):518-530. doi: 10.1002/jor.25758. Epub 2023 Dec 16. PMID: 38102985; PMCID: PMC10932846.
Bone reconstruction
Putzer, David; Tapeanu, Gabriela; Shahriary, Fatemeh; Guarino, Roberto; Thaler, Martin; Nogler, Michael; Awaja, Firas: A Clinical Investigation of Hip Implant Migration and Wear.
BIOMEDICAL MATERIALS AND DEVICES. 2025 accepted for publication on 31.01.2024 doi: 10.1007/s44174-024-00273-2.
Egger, Valentina; Dammerer, Dietmar; Degenhart, Gerald; Pallua, Johannes D.; Schmölz, Werner; Thaler, Martin; Kühn, Klaus-Dieter; Nogler, Michael; Putzer, David: Does the Addition of Low-Dose Antibiotics Compromise the Mechanical Properties of Polymethylmethacrylate (PMMA)?
POLYMERS. 2024; 16(16); 2378. PubMed: 39204597 doi: 10.3390/polym16162378 [FLDID: 142904] IF: 4.700 (2023) / ZIT: 1
Putzer, D.; Egger, V.; Pallua, J.; Thaler, M.; Schmölz, W.; Nogler, M.: Different polymethylmethacrylate (PMMA) reinforcement strategies for long bone osteoplasty procedures: a controlled laboratory comparison using the 4-point bending test.
BMC MUSCULOSKELETAL DISORDERS. 2024; 25(1); 1058. PubMed: 39709357 doi: 10.1186/s12891-024-08148-9 [FLDID: 144013] IF: 2.200 (2023) / ZIT: 0
Putzer, D.; Pallua, J.; Degenhardt, G.; Dammerer, D.; Nogler, M.; Arora, R.: Microarchitectural properties of compacted cancellous bone allografts: A morphology micro-computed tomography analysis. JOURNAL OF THE MECHANICAL BEHAVIOR OF BIOMEDICAL MATERIALS. 2024; 160; 106781.
PubMed: 39426354 doi: 10.1016/j.jmbbm.2024.106781 [FLDID: 143364] IF: 3.300 (2023) / ZIT: 0
Putzer, D.; Schroeder, L.; Wassilew, G.; Liebensteiner, M.; Nogler, M.; Thaler, M.: Early Learning Curve in Robotic-Assisted Total Knee Arthroplasty: A Single-Center Experience.
JOURNAL OF CLINICAL MEDICINE. 2024; 13(23); 7253.
PubMed: 39685712 doi: 10.3390/jcm13237253 [FLDID: 144273] IF: 3.000 (2023) / ZIT: 0
Selection of Funding
Clinical Studies:
AgNovos Healthcare Sponsoring
IIT Grant DePuySynthes/Johnson&Johnson
IIT Grant Medacta International
IIT Grant Medartis
AO Foundation
Zimmer Biomet, Zug, Switzerland
Verkehrssicherheitsfond – Land Tirol
Biomechanic Lab:
MedTech Industry
Tiroler Wissenschaftsfond
AO Trauma Austria
Biofilm Lab:
2023 – Ongoing – AdvantIQX – DIAMOND – Germany
2023 – 2024 – Bachmann – Innsbruck – Gynecology
2023 – 2024 – CTB-A Krems an der Donau – Austria
2024 – 2025 – CTB-A Krems an der Donau – Austria
2024-2025 – Land Tirol Funds – CarbonCompetence, Wattens Austria
2025 – Heraeus Medical – DAIR Phase I
microCT/3D Reconstruction:
AO-Trauma; Enhancing Bone Infection Diagnosis with Hyperspectral Imaging: Pathogen Discrimination and Diagnostic Potential“ (i-med GZ: 87027)
SpecOps – Spektroskopische Methoden zur Feststellung von Kontaminationsrückständen auf Kleidung und Personen“ (i-med GZ: 87027)
Der Vogel aus dem Eis: VIRTUAL REALITY-Expedition zur Gletschermumie“ (P7250-013-058).
Collaborations
Clinical Studies:
Department of Otorhinolaryngology Innsbruck
Department of Physical Therapy Innsbruck
Medartis AG, Basel, Switzerland
Medacta International SA, Castel San Pietro, Switzerland
Zimmer Biomet GmbH, Zug, Switzerland
Biomechanic Lab:
Prof Dr. Alexander Disch, University Comprehensive Spine Center, Institute University Center for Orthopaedics, Trauma and Plastic Surgery, University Hospital Carl Gustav Carus, TU Dresden, Germany
PD Dr. Michael Schlumberger, Klinikum Stuttgart, Germany
Prof. Dr.-Ing. Sebastian Dendorfer and Prof. Dr.-Ing Lars Krenkel, OTH Regensburg, Germany
Dr. Karsten Große, SRH Waldklinikum Gera, Germany
Dr. Robin Srour, Hopitaux Civils de ColmaR; France
Biofilm Lab:
AdvantIQX, Augsburg, Germany
Cell and Tissue Banking, Krems an der Donau, Austria
CarbonCompetence, Wattens, Austria
Medel, Innsbruck, Austria
PJI Consensus Group, 1400 professionals worldwide dealing with Musculoskeletal Infections
Bone Reconstruction:
Stryker Robotics, Freiburg, Germany
Politecnico di Torino, Torino, Italy
Krems University Hospital, Krems, Austria
Institute of Medicine, Suranaree University of Technology, Thailand
Specto Medical, Basel, Switzerland
microCT/3D Reconstruction:
Institute of Pathology, Neuropathology and Molecular Pathology
Institute of Hygiene and Medical Microbiology
Institute of Legal Medicine
OrganLifeTM, Department of Visceral, Transplant and Thoracic Surgery
Institute of Analytical Chemistry and Radiochemistry
Leibniz Institute of Photonic Technology (Leibniz-IPHT), Jena, Germany
Westcam, Austria