
Anichstraße 35
6020 Innsbruck
Fax: +43 (0)50 504 23317
Email: guenter.weiss@i-med.ac.at
Website: https://inneremed2.tirol-kliniken.at/ _
Research year
Research Branch (ÖSTAT Classification)
301902, 302030, 302068, 302072, 301302, 301303, 303031, 303041
Keywords
anaemia research, Clinical and experimental infectious diseases, clinical immunology, diagnostic biomarkers, experimental and clinical pneumology, host-pathogen interaction, iron homeostasis, lipid metabolism, personalised medicine, and rheumatology
Research Focus
Our research is centred on two key areas: the identification of fundamental principles and the development of effective clinical management strategies for diseases. We achieve this by integrating scientific research with clinical practice, ensuring a comprehensive understanding of the biochemical, molecular and translational aspects of diseases. Our clinical expertise lies in internal medicine, with a focus on infectious diseases, immunology, pneumology, rheumatology and general internal medicine.
General Facts
The department focuses on all clinical and scientific aspects of infectious diseases (ID), clinical immunology, pneumology and rheumatology.
Our clinic is a referral centre with special in-patient and outpatient facilities for ID, pneumology and rheumatology. It is home to a diagnostic laboratory for ID and rheumatology. We also operate facilities for ultrasound, echocardiography, bronchoscopy and pulmonary function testing. We comprehensively cover all general aspects of internal medicine and understand the interconnections with all related medical disciplines.
Our scientific research groups are equipped with a strong background in cellular and molecular biology as well as immunology, using the latest analytical techniques. We have elucidated numerous mechanisms, diagnostic algorithms and novel therapeutic principles in a variety of diseases, including anaemia of inflammation, atherosclerosis, iron dyshomeostasis, infections with extracellular and intracellular microbes, host-pathogen interplay, and the interconnections of iron, energy and lipid metabolism with the immune system. In our research laboratories and on our clinical wards, we investigate relevant topics in clinical ID, rheumatology and pneumology by using a wide range of up to date in silico, in vitro and in vivo technologies. We have established registries and biobank sampling for common and rare diseases in clinical research in ID, pneumology and rheumatology. This allows us to better understand the specific course of various diseases in our fields of expertise. It also helps to identify clinical-relevant disease patterns and novel biomarkers in order to established optimised therapies for our patients.
Research
Our research groups are dedicated to translating molecular findings from clinical samples and disease models into better diagnostic and therapeutic tools. Our objective: to improve the care of patients with infectious, inflammatory, metabolic, pulmonary and rheumatic diseases. Our research groups possess a strong research background in iron homeostasis, immunology, lipid metabolism and translational medicine. Our combined research efforts focus on the complex interplay of iron and cholesterol handling in infectious, inflammatory and metabolic diseases, the pathophysiology and therapy of anaemia of inflammation (AI), the improvement of rheumatic disease classification and the clinical management of patients with pulmonary diseases. The recent pandemic lead to a focus on clinical and experimental research linked to SARS-CoV2 and its sequels, which built up on expertise and previous studies on diseases pattern related to influenza and bacterial pneumonia.
Infectious Diseases – From Bacteria to Respiratory Viruses and the role of nutritional immunity
Infectious agents are fought off by the concerted action of innate and acquired immune mechanisms. A central feature of the innate immune response to pathogens, including viruses, bacteria and fungi, is the sequestration of nutrients such as iron and lipids. “Nutritional immunity” is the mechanism of nutrient withdrawal from microbes, iron is a major target of the nutritional immune response because it plays an essential role in the growth and proliferation of microbes.
Disorders of iron homeostasis are both frequent and important to the clinical course and outcome of underlying and coexisting diseases. They are also relevant for global public health. Low-income countries have a particularly high prevalence of iron deficiency anaemia, affecting both children and adults. However, region-wide oral iron supplementation in these countries has resulted in higher rates of severe infection, for reasons that remain largely unknown. Our research focuses squarely on the impact of iron supplementation on the immune system and the course of bacterial infections. Our research shows that iron supplementation fuels the proliferation of Salmonella Typhimurium in macrophages (Fig. 1). We employed a mouse model mimicking iron deficiency anaemia and normal iron status and supplemented iron prior to infection with Salmonella. Studies show that individuals with iron deficiency who are supplemented with iron have an impaired infection control and succumb faster to death. This is on the one hand due to increased iron availability for bacteria and on the other hand related to reduced neutrophil function and CD8+ T- cell responses in iron deficient, anaemic mice. Such studies have a major impact on preventive iron supplementation programmes in low-income countries.
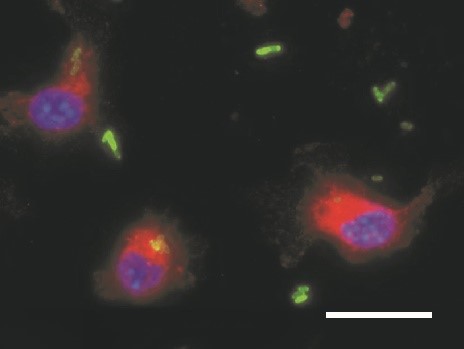
We are also determined to understand how iron is compartmentalised in the host organism during infections and which iron transporters and proteins are essential to control iron delivery to microbes. Specifically, different mechanisms exist to withhold iron from extracellular pathogens, such as E. coli and Aspergillus fumigatus. In contrast, no such mechanisms exist to combat intracellular pathogens, such as viruses, Chlamydia, Listeria or Salmonella species. The mechanisms involved must be adapted and fine-tuned, depending on the type and location of the noxious agent confronting the immune system. Delineating the signalling and metabolic pathways from pathogen or danger recognition to iron sequestration will allow us to characterise relevant mechanisms and to identify drugable targets for therapeutic intervention.
We discovered that patients with sepsis who had elevated levels of the iron storage protein ferritin in their plasma died less often. We found clear evidence of a link between higher plasma iron and ferritin levels and increased mortality. We have also demonstrated that in subjects with hemoglobinopathies resulting in sustained iron overload, the degree of iron loading determines the course of infections and the response to vaccination. Our results clearly demonstrate that iron availability affects both innate and adaptive immune responses to microbes and thus the course of infectious diseases.
Iron availability affects not only innate immune response and macrophage polarisation. We have shown that it also orchestrates T-helper cell differentiation by regulating the expression of immune checkpoint regulators TIM-3. Studies are investigating the effects of immune cell polarisation by cytokines on immune effector functions in models of bacterial infection. Analyses are focusing on how amino acid metabolisms with a particular emphasis on arginine, tryptophan, glutamate and tyrosine, affect innate immune response and/or microbial survival. We are analysing the metabolic effects of iron on TCA cycle activity and mitochondrial respiration in inflammatory conditions. This will provide us with more insights into the complex regulatory network of immune metabolism in infection. Our ongoing research applications are focused on studying these networks in the tumour microenvironment and revealing the link between iron availability and cancer biology and host immune responses.
As part of a collaborative network at the MUI (Z.Trajanoski, Bioinformatics; D.Wilflingseder/W.Posch, Microbiology and Hygiene), we study innate host resistance mechanism to infection employing lung organoid models. This enables us to investigate the immediate response of host epithelial cells to infections with bacteria (such as Pseudomonas aeruginosa) or viruses SARS-CoV2) and how pathogens manipulate the host’s response to gain invasiveness.
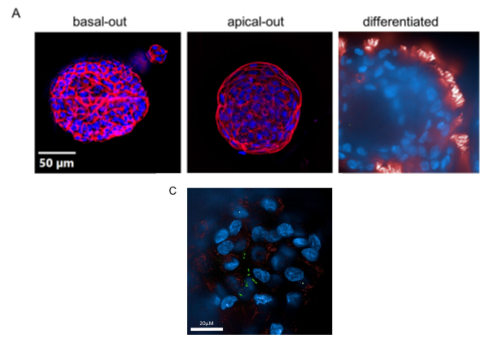
We have investigated multiple clinical aspects of respiratory viral and bacterial infections over many years, with a focus on influenza and pneumococcal/legionella pneumonia. The emergence of the COVID-19 pandemic directed our scientific interest to this infection. We studied the association of anaemia and disturbances of iron homeostasis with the course of this disease. We also identified and validated biomarkers and diagnostic tests for the identification and prediction of the COVID-19 progression. Furthermore, we comprehensively assessed organ involvement, focusing on lungs and heart, and the course of the disease in patients with respiratory infections over time. We conducted a prospective study to investigate immune responses to vaccination against SARS-CoV2 and influenza in immune-compromised patients and the impact of modifying factors on B- and T- cell-mediated immune protection. We are conducting translational studies to gain a better understanding of the pathological traits underlying post-acute infection syndromes (including long COVID). Our research focuses on subclinical inflammation, altered metabolic pathways (including mitochondrial function) and microbiota functionality.
Iron metabolism and Anaemia research
Anaemia of inflammation (AI) is a prime example of the link between iron homeostasis and inflammation. Iron storage in macrophages is a major contributing factor in the pathogenesis of AI and renders iron unavailable for haemoglobin synthesis and red blood cell production. We have identified crucial pathways leading to AI. These pathways focus mainly on the effects of cytokines on iron homeostasis in macrophages. We have also elucidated the complex regulatory interactions of iron homeostasis with central innate immune effector pathways. These include the activity and formation of the iron hormone hepcidin. Recently, we have conducted a comprehensive investigation into the potential of oral and intravenous iron supplementation to treat AI in various disease models, including AI or chronic renal anaemia. This investigation has yielded significant new insights regarding the therapeutic efficacy of these treatments and potential off-target effects. Our data shows that proper differential diagnosis between AI and AI with true iron deficiency (AI+IDA) is crucial. The reason is clear: c responses to iron supplementation differ significantly between these entities. In collaboration with the Department of Cardiology, we investigated a cohort of patients with acute and chronic heart failure, where iron deficiency is common in order to address the needs of iron in these patients and the applicability of current treatment guidelines to clinical practice and efficacy. We investigated organ iron stores in subjects with AI and AI+IDA and compared the results to established serum markers of iron homeostasis and erythropoiesis. This study revealed that AI resulted in tissue iron retention, whereas AI +IDA was associated with low iron levels in the spleen, liver and heart, indicating a potential response to iron supplementation therapy.
Together, our studies continue to identify links between inflammation, iron homeostasis, and disease outcome for a better understanding of the pathophysiological mechanisms, and for improving the diagnosis and targeted therapy of patients with inflammatory anaemia.
Moreover, in collaboration with the Department of Neurology, we are investigating the interaction between iron dyshomeostasis and neurological disorders, focusing on restless legs syndrome (RLS), Parkinson’s disease (PD) and Friedreich ataxia (FA). We have previously discovered that dopamine acts as an iron chaperone, determining the delivery of iron to cells and mitochondria, which could be important for the pathogenesis of RLS and PD. However, toxic accumulation of iron in mitochondria appears to be important in FA. Combining in vitro and clinical data analysis will help us better understand the pathophysiological abnormalities of these diseases and how modifying iron homeostasis may affect their clinical course.
Rheumatological Diseases
We are particularly interested in innate immunity and its role in rheumatic diseases. In this context, we are interested in establishing links between iron metabolism, innate immunity and rheumatological disease models. One highly interesting area in which the ‘bedside to bench’ scientific approach can be applied is VEXAS syndrome, a recently discovered autoinflammatory disease. Some aspects of this disease are known, which is caused by different somatic mutations in the UBA1 gene on the X chromosome. However, the exact pathophysiology of how this mutation, which mainly occurs in myeloid cells, causes severe hyperinflammation remains unclear. Alongside a cell culture model, we are processing patient samples using the latest techniques to improve our standing of the pathophysiology and potentially discover targeted therapeutic approaches.
Another area of our research focuses on gout. In particular, we are collaborating with our radiology colleagues on new imaging techniques, such as dual energy CT (DECT). This non-invasive method can detect monosodium urate crystals deposits near the joints, which significantly aids the diagnosis of gout. It has recently been reported that these deposits can also be found in the cardiovascular system. Further studies are now needed to establish whether this constitutes an independent cardiovascular risk factor.

One key area of focus is the clinical, imaging, and immune-phenotypic characterisation of age-related chronic inflammatory rheumatic diseases, with a particular focus on polymyalgia rheumatica (PMR), giant cell arteritis (GCA) and elderly-onset rheumatoid arthritis (EORA).
Recent efforts have focused on evaluating the reliability of the newly developed OMERACT Giant Cell Arteritis Ultrasound Score (OGUS), driven by a longstanding interest in integrating ultrasound into routine clinical rheumatology practice. This assessment was conducted in a patient-based study involving both vascular ultrasonography experts and non-experts. In a collaborative study with the OMERACT US LVV Task Force (convenors: Prof. C. Dejaco (MD/PhD, Italy) and Prof. W. Schmidt, (MD, Germany)), Duftner et al. demonstrated fair to moderate inter-reader reliability and good intra-reader reliability for OGUS, the Southend score, and Halo count among vascular ultrasound experts. Notably, the inter-reader reliability of non-experts significantly improved following 90 minutes of theoretical training and 240 minutes of practical training.
Additionally, ongoing OMERACT GCA ultrasound projects are investigating the potential of 3D-printed phantoms and artificial intelligence for use in diagnosing GCA.
Dr. Duftner has contributed to the development of treat-to-target recommendations for managing polymyalgia rheumatica (PMR) and GCA, and to the updating of the EULAR guidelines for managing large vessel vasculitis.
Another significant area of interest is the characterisation of disease risk parameters for the development of psoriatic arthritis in patients with psoriasis. In this context, the STOP-PsA study has been implemented at the Medical University of Innsbruck. This study focuses on imaging, cardiovascular parameters and the role of microRNAs (µRNAs) in the early detection of psoriasis patients at risk of transitioning to psoriatic arthritis. The latter study was awarded by the Austrian Society of Rheumatology and Rehabilitation; Dr. Manger served as the grant applicant and Principal Investigator and Dr. Duftner as the Principal Investigator.
In the field of rare diseases, Dr. Duftner contributed to the development of management recommendations for sarcoidosis, a condition with diverse manifestations and limited evidence-based treatment options.
Like many medical specialties, rheumatology is facing a shortage of specialists, which threatens the sustainability of institutions dedicated to rheumatological care. The commitment of medical professionals to their institutions is crucial and hinges on the creation of an equitable work environment. Further insight into the factors that influence physician commitment is required to achieve equity in rheumatology and other medical fields, a gap that remains largely unexplored.
The increasing demand for rheumatological care, coupled with an insufficient number of young professionals entering the field poses a significant challenge to the future of rheumatology. In response, there are ongoing efforts to improve rheumatology education and develop the future workforce.
Pneumology
Over the past two years, our department has spearheaded a comprehensive portfolio of research into the effects of the SARS-CoV-2 virus, which seamlessly integrates with our broader work within the COVIhge (the COVI-19 Human Genetic Effort () Network. We have delved deep into the molecular underpinnings of severe viral infections, uncovering inborn errors in type I interferon pathways that predispose patients to life‐threatening outcomes. Our investigations into innate immunity have revealed that SARS‑CoV‑2 robustly engages TLR4, driving hyperinflammation via the canonical MyD88–NF‑κB signalling pathway in human macrophages. This finding enriches our understanding of SARS-CoV-2 infection and paves the way for targeted TLR4-directed therapies that act on TLR4. Our work on these genetic vulnerabilities complements the extensive body of publications within the COVDhge Network, emphasising the need for precision medicine in managing severe viral infections.
In parallel, our collaborative work with the Department of Cardiothoracic Surgery resulted in the groundbreaking discovery of biglycan as the second endogenous ligand of Toll‑Like Receptor 3 (TLR3) in animal models. This seminal study, published in Circulation2 and highlighted in Science, elucidates the BGN–TLR3–IFNAR1 axis as a critical regulator of calcific aortic valve disease. It also shows how aberrant type I interferon signalling, intertwined with immunosenescene processes, contributes to severe viral infections and valvular pathology.
Our research into the effects of the SARS-CoV-2 virus extends beyond basic science into clinical applications. We have developed and validated diagnostic and therapeutic strategies to address both acute infection and its long-term consequences, and our prospective registries provide invaluable insights into patient recovery trajectories. Integrating this clinical work with our molecular findings reinforces our commitment to improving patient outcomes.
A hallmark defining feature of our approach is the growing integration of artificial intelligence (AI) in our studies. We use cutting-edge machine learning algorithms to assess the extent of disease quantitatively and track clinical progress. These tools are proven invaluable for predicting patient outcomes, optimising risk stratification, and identifying distinct phenotypic clusters. By fusing advanced analytics with clinical insights, we ensure that our research remains at the very forefront of modern pulmonary medicine.3
Moreover, our ongoing studies, such as COLIPRIS and REASSESS, exemplify the unique, multidisciplinary collaboration at our clinic, where infectious diseases, rheumatology and respiratory medicine work closely together. By leveraging our extensive collective expertise, we are addressing fundamental questions regarding imaging modalities, highly variable clinical courses and immunological disease markers. Advanced AI methodologies further enhance our ability to analyse these parameters, with the potential to identify predictive factors that could transform risk stratification and patient management in systemic sclerosis and rheumatoid arthritis.
In summary, our interdisciplinary approach over the past two years, which has bridged the fields of molecular immunology, clinical investigation and computational innovation, has advanced our understanding of both severe viral infections and lung fibrosis, and is also paving the way for groundbreaking therapies. Our ongoing commitment to using AI in clinical studies demonstrates our dedication to predicting and improving patient outcomes, ensuring that our research remains as innovative and responsive as the challenges we face.
Pictures
Selected Publications
Infectious/Inflammatory Diseases and Immune-metabolism
Grubwieser P, Böck N, Soto EK, Hilbe R, Moser P, Seifert M, Dichtl S, Govrins MA, Posch W, Sonnweber T, Nairz M, Theurl I, Trajanoski Z, Weiss G. Human airway epithelium controls Pseudomonas aeruginosa infection via inducible nitric oxide synthase. Front Immunol. 2024 Dec 3;15:1508727. doi: 10.3389/fimmu.2024.1508727. PMID: 39691712; PMCID: PMC11649544.
Gietl M, Burkert F, Hofer S, Gostner JM, Sonnweber T, Tancevski I, Pizzini A, Sahanic S, Schroll A, Brigo N, Egger A, Bellmann-Weiler R, Löffler-Ragg J, Weiss G, Kurz K. Laboratory parameters related to disease severity and physical performance after reconvalescence of acute COVID-19 infection. Sci Rep. 2024 May 6;14(1):10388. doi: 10.1038/s41598-024-57448-6. PMID: 38710760
Burkert FR, Oberhollenzer M, Kresse D, Niederreiter S, Filippi V, Lanser L, Weiss G, Bellmann-Weiler R. Cardiac Damage in Patients Infected with Different SARS-CoV-2 Variants of Concern. Microorganisms. 2024 Dec 18;12(12):2617. doi: 10.3390/microorganisms12122617. PMID: 39770819; PMCID: PMC11676750.
Sukhbaatar N, Schöller M, Fritsch SD, Linke M, Horer S, Träger M, Mazić M, Forisch S, Gonzales K, Kahler JP, Binder C, Lassnig C, Strobl B, Müller M, Scheiber-Mojdehkar B, Gundacker C, Dabsch S, Kain R, Hengstschläger M, Verhelst SHL, Weiss G, Theurl I, Weichhart T. Duodenal macrophages control dietary iron absorption via local degradation of transferrin. Blood 2023 Jun 8;141(23):2878-2890. PMID: 37018657
Ghoti H, Zreid H, Ghoti I, Bourgonje AR, Diepstra A, van Goor H, Avivi I, Jeadi H, van Eijk LE, Weiss G. Clinical outcome and humoral immune responses of β-thalassemia major patients with severe iron overload to SARS-CoV-2 infection and vaccination: a prospective cohort study. EClinicalMedicine 2023 Jul 26;62:102096. doi: 10.1016/j.eclinm.2023.102096. eCollection 2023 Aug.
Iron Homeostasis and Anemia Research
Valente De Souza L, Hoffmann A, Fischer C, Petzer V, Asshoff M, Theurl I, Tymoszuk P, Seifert M, Brigo N, Hilbe R, Demetz E, Von Raffay L, Berger S, Barros-Pinkelnig M, Weiss G. Comparative analysis of oral and intravenous iron therapy in rat models of inflammatory anemia and iron deficiency. 2023 Jan 1;108(1):135-149.PMID: 35796011
Lanser L, Plaikner M, Schroll A, Burkert FR, Seiwald S, Fauser J, Petzer V, Bellmann-Weiler R, Fritsche G, Tancevski I, Duftner C, Pircher A, Seeber A, Zoller H, Kremser C, Henninger B, Weiss G. Tissue iron distribution in patients with anemia of inflammation: Results of a pilot study. Am J Hematol. 2023 Jun;98(6):890-899. PMID: 36880875
Grander M, Haschka D, Indelicato E, Kremser C, Amprosi M, Nachbauer W, Henninger B, Stefani A, Högl B, Fischer C, Seifert M, Kiechl S, Weiss G, Boesch S. Genetic Determined Iron Starvation Signature in Friedreich’s Ataxia. Mov Disord. 2024 Jul;39(7):1088-1098. doi: 10.1002/mds.29819. Epub 2024 Apr 30. PMID: 38686449
Lanser L, Poelzl G, Messner M, Ungericht M, Zaruba MM, Hirsch J, Hechenberger S, Obersteiner S, Koller B, Ulmer H, Weiss G. Imbalance of Iron Availability and Demand in Patients With Acute and Chronic Heart Failure. J Am Heart Assoc. 2024 May 7;13(9):e032540. doi:10.1161/JAHA.123.032540. Epub 2024 Apr 19. PMID: 38639356
Buoso C, Seifert M, Lang M, Griffith CM, Talavera Andújar B, Castelo Rueda MP, Fischer C, Doerrier C, Talasz H, Zanon A, Pramstaller PP, Schymanski EL, Pichler I, Weiss G. Dopamine‑iron homeostasis interaction rescues mitochondrial fitness in Parkinson’s disease. Neurobiol Dis. 2024 Jun 15; 196:106506. doi: 10.1016/j.nbd.2024.106506. Epub 2024 Apr 21. PMID: 38648865
Rheumatology
Klauser AS, Halpern EJ, Strobl S, Gruber J, Feuchtner G, Bellmann-Weiler R, Weiss G, Stofferin H, Jaschke W. Dual-Energy Computed Tomography Detection of Cardiovascular Monosodium Urate Deposits in Patients With Gout. JAMA Cardiol. 2019 Oct 1;4(10):1019-1028. doi: 10.1001/jamacardio.2019.3201.
Held J, Schwabl C, Haschka D, Maier S, Feuchtner G, Widmann G, Duftner C, Weiss G, Klauser A. Major cardiovascular events in patients with cardiovascular monosodium urate deposits in atherosclerotic plaques. Rheumatology (Oxford). 2024 Apr 23:keae240. doi: 10.1093/rheumatology/keae240. Epub ahead of print.
Dejaco C, Kerschbaumer A, Aletaha D, et al. “Treat-to-Target Recommendations in Giant Cell Arteritis and Polymyalgia Rheumatica,” Ann Rheum Dis., 2024 Jan 2;83(1):48-57. doi: 10.1136/ard-2022-223429.
Dejaco C, Ramiro S, Bond M, et al. “EULAR Recommendations for the Use of Imaging in Large Vessel Vasculitis in Clinical Practice: 2023 Update,” Ann Rheum Dis., 2024 May 15;83(6):741-751. doi: 10.1136/ard-2023-224543
Duftner C, Redlinger N, Bruyn GA, et al. “Reliability of the OMERACT Giant Cell Arteritis Ultrasound Score (OGUS): Results of a Patient-Based Exercise Involving Experts and Non-Experts in Vascular Ultrasonography,” RMD Open, 2025 Jan 2;11(1):e004667. doi: 10.1136/rmdopen-2024-004667
Pneumology
Sahanic S, Hilbe R, Dünser C, Tymoszuk P, Löffler-Ragg J, Rieder D, Trajanoski Z, Krogsdam A, Demetz E, Yurchenko M, Fischer C, Schirmer M, Theurl M, Lener D, Hirsch J, Holfeld J, Gollmann-Tepeköylü C, Zinner CP, Tzankov A, Zhang SY, Casanova JL, Posch W, Wilflingseder D, Weiss G, Tancevski I. SARS-CoV-2 activates the TLR4/MyD88 pathway in human macrophages: A possible correlation with strong pro-inflammatory responses in severe COVID-19. Heliyon. 2023 Nov 17;9(11):e21893. doi: 10.1016/j.heliyon.2023.e21893.
Gollmann-Tepeköylü C, Graber M, Hirsch J, Mair S, Naschberger A, Pölzl L, Nägele F, Kirchmair E, Degenhart G, Demetz E, Hilbe R, Chen HY, Engert JC, Böhm A, Franz N, Lobenwein D, Lener D, Fuchs C, Weihs A, Töchterle S, Vogel GF, Schweiger V, Eder J, Pietschmann P, Seifert M, Kronenberg F, Coassin S, Blumer M, Hackl H, Meyer D, Feuchtner G, Kirchmair R, Troppmair J, Krane M, Weiss G, Tsimikas S, Thanassoulis G, Grimm M, Rupp B, Huber LA, Zhang SY, Casanova JL, Tancevski I, Holfeld J.Toll-Like Receptor 3 Mediates Aortic Stenosis Through a Conserved Mechanism of Calcification Circulation. 2023 May 16;147(20):1518-1533. doi: 10.1161/CIRCULATIONAHA.
Sahanic S, Tymoszuk P, Luger AK, Hüfner K, Boehm A, Pizzini A, Schwabl C, Koppelstätter S, Kurz K, Asshoff M, Mosheimer-Feistritzer B, Coen M, Pfeifer B, Rass V, Egger A, Hörmann G, Sperner-Unterweger B, Helbok R, Wöll E, Weiss G, Widmann G, Tancevski I, Sonnweber T, Löffler-Ragg J. COVID-19 and its continuing burden after 12 months: a longitudinal observational prospective multicentre trial. ERJ Open Res. 2023 Mar 13;9(2):00317-2022. doi: 10.1183/23120541.00317-2022.
Selection of Funding
- Christian Doppler Society, Christian Doppler Laboratory for Iron Metabolism and Anemia research, Günter Weiss
- Austrian Research Funds (FWF)-Doctoral Programme (HOROS—W-1253), Günter Weiss
- Austrian Research Funds (FWF) Doc-Fund project-82 (Cellular Basis of Disease), Günter Weiss
- Tyrolean research Funds- Long Covid and ME/CFS Foundation, Katharina Kurz
- ÖGR Research Grant- Lukas Lanser
- ÖGR Research Grant- Eva Manger
- TWF Grant Clemens Gehrer
- TWF Grant Manuel Grander
- TWF Grant Sabina Sahanic
Collaborations
- Christian Bogdan, Institute of Clinical Microbiology, Immunology and Hygiene, Friedrich-Alexander-Universität Erlangen-Nürnberg, Erlangen, Germany
- Dirk Bumann, Biocenter, Basel, Schweiz
- Jean-Laurent Casanova, Lab of Genetics of Human Infectious Diseases, Rockefeller University, New York, USA
- Christian Dejaco, Department of Rheumatology, Hospital of Brunico (SABES-ASDAA), Bruneck, Italy
- Isabella Grabner, Institute for Strategy and Managerial Accounting, Vienna University of Economics and Business Administration, Vienna, Austria
- Jonathan Jantsch, Inst. für med. Mikrobiologie und Immunologie, Univ. Köln, D
- Martina U. Muckenthaler, Department of Pediatric Hematology, Oncology and Immunology, University of Heidelberg, Heidelberg, Germany
- Giuseppe Paglia, University of Milan, Italy
- Irene Pichler, EURAC, Bolzano, Italy
- Miguel Soares, Institute Gulbenkain di Science, Lisboa, Portugal
- Thomas Weichart, Institute of Genetics, University of Vienna, A