Anichstraße 35
6020 Innsbruck
Fax: +43 (0)50 504 23002
Email: Gudrun.Ratzinger@i-med.ac.at
Website: https://dermatologie.tirol-kliniken.at/
Research year
Research Branch (ÖSTAT Classification)
301902, 302002, 302011, 302055, 302087
Keywords
allergy, dendritic cells, epidermal barrier, Immunology, infectious diseases including HIV, Inflammation, Keratinocytes, photobiology, skin cancer, and venereology
Research Focus
In the dermatology department, basic research and patient care are intimately interconnected. This will ultimately be advantageous to our patients, who will benefit from new diagnostic and therapeutic approaches. Our interconnected research programme covers tumour biology, the cutaneous immune system, allergies, autoimmunity, inflammatory and infectious skin diseases, HIV infection, venereology, genetic skin diseases, photobiology and dermatohistopathology.
General Facts
The Department is one of the oldest and internationally most renowned dermatology departments in the German-speaking countries. It sees approximately 3,000 inpatients and 50,000 outpatients every year and focuses on complex dermatovenereology and allergy with a strong track record in translational research.
Within the main research areas of the university, the department conducts researchs on: 1) infection immunity and transplantation; 2) oncology, including tumour immunology; 3) genetics, epigenetics and genomics of epidermal barrier disorders; and 4) inflammation, including translational research on atopic dermatitis, which is the most common chronic inflammatory skin disease worldwide.
- HIV patients are studied in a national OEHIVKOS cohort, which was initiated in and is run from Innsbruck, and which is well connected with international registries.
- The Department is certified both by Quality Austria (ISO 9001) and by OnkoZert (German Cancer Society). Research programmes are coordinated by the Austrian oncology group (ÖGDV) and the melanoma consortium MEMS (EU Interreg).
- The Department is a designated centre of expertise (Type B centre) and a European Reference Network (ERN) for genodermatoses, with a focus on epidermal differentiation disorders (EDD).
Research
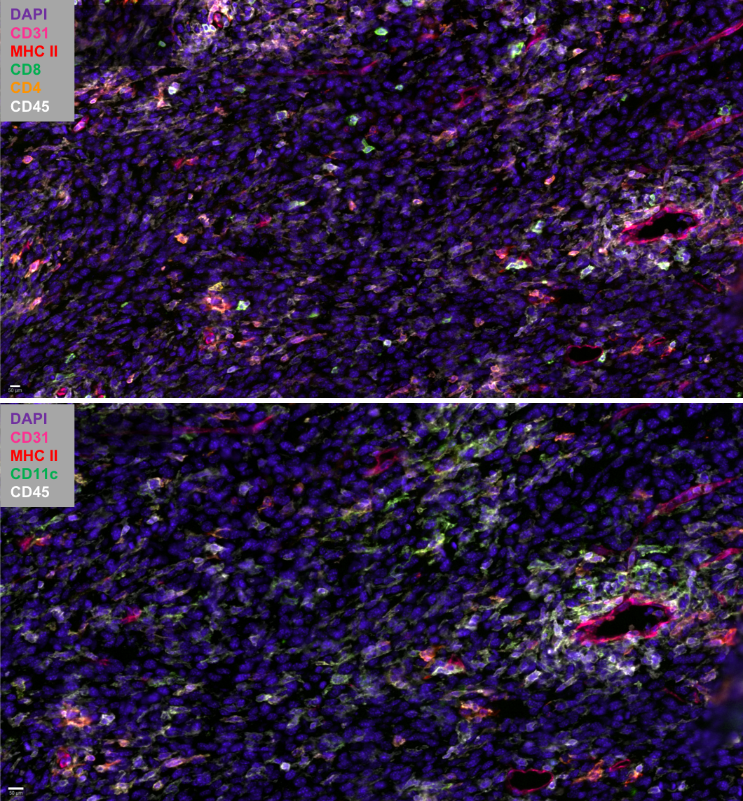
Dendritic cells in tumour immunity and autoimmunity – potential application for immunotherapy
Patrizia Stoitzner, Christoph Tripp, Claudia Zelle-Rieser, Helen Strandt, Sophie Dieckmann, Janine Vierthaler, Christina Mayerl
Dendritic cells (DC) act as sentinels of the skin immune system and this unique ability renders them optimal targets for immunotherapy of various skin diseases, such as melanoma and autoimmunity (e.g. vitiligo). To improve DC-based immunotherapy, we need to understand how skin DC are affected by tumour development and how they are involved in the induction of autoimmunity. With the help of mouse models for melanoma and vitiligo, we are investigating the functional role of the various skin DC subsets in T cell responses. The results will help us develop skin immunization strategies to treat cancer and potentially also vitiligo. We are also working on targeting antigens to skin DC subsets with the help of nucleotide-based vaccine platforms in mouse and human skin models. As the future of cancer treatment lies in combination therapy, we are evaluating the potential of DC-based immunotherapy together with tumour-targeted therapy, e.g. BRAF/MEK-inhibitor therapy. In collaboration with the 3D-bioprinting lab at the MUI we are developing alternatives to animal experiments, such as immunocompetent 3D-bioprinted skin/melanoma-on chip-models. Such models will allow live-cell imaging of DCs interacting with tumour cells during tumour-targeted therapy. Our diverse model systems will enable us to unravel the cellular crosstalk between DC subtypes, tumour/skin stromal cells and T cells in the peripheral tissue with the ultimate goal of advancing therapeutic strategies.
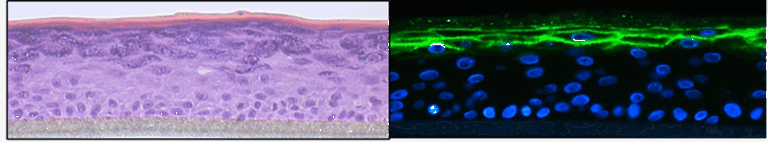
Atopic dermatitis
Sandrine Dubrac, Verena Moosbrugger-Martinz
Atopic dermatitis is the most common chronic inflammatory skin disease worldwide with a yearly prevalence of up to 30 % in children and 20 % in adults. It is characterized by Th2 inflammation over dry skin and is associated with comorbidities that lead to a significant disease burden on patients and societies. We are working towards a better understanding of the pathogenesis of the condition. Our holistic approach includes metabolic abnormalities, skin microbiota and systemic immune dysfunctions. An essential tool of our research is high-quality 3D organotypic cultures, which we generate from patients’ skin cells. We have recently disproven the paradigm that all patients with atopic dermatitis are superinfected with Staphylococcus aureus. In a further project we are elucidating the intertwined relationship between atopic dermatitis and fungal skin microbiota. We were the first to decipher the role of the pregnane x receptor (PXR), a master regulator of xenobiotic metabolism, in the skin, showing that overexpression of PXR in the epidermis leads to atopic dermatitis–like symptoms. Our recent findings point to a major role for PXR in the skin’s response to environmental pollutants, such as endocrine disruptors. We have demonstrated the central role of oxidative stress in atopic dermatitis, via altered mitochondrial function, offering new avenues for therapeutic intervention. We are currently deciphering the molecular pathomechanisms of the comorbidities in atopic dermatitis, psoriasis and ichthyoses with a focus on neuropsychological disorders, Finally, we are undertaking a translational research project to develop novel therapeutic strategies to treat inflammatory skin diseases (patent EP22187107.2).
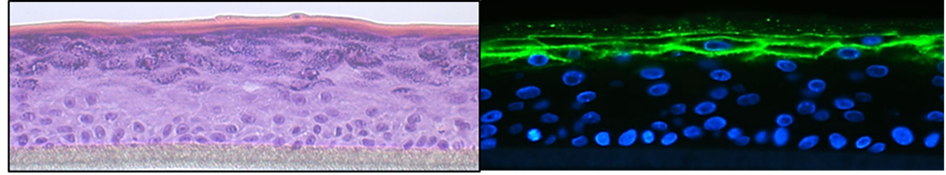
Skin barrier function and inflammation: the role of the microbiome
Verena Moosbrugger-Martinz
The skin microbiome is altered in diseases with chronic skin inflammation and barrier dysfunction and the changes may exacerbate skin disease. While bacteria such as Staphylococcus aureus are well studied, little is known about the fungal microbiota and the mechanisms by which they affect the cutaneous immune system and skin barrier integrity and function. We are working towards closing this gap in our knowledge. We are also addressing the effect of anti-inflammatory systemic therapies on skin inflammation and barrier dysfunction, particularly in patients with ichthyosis.
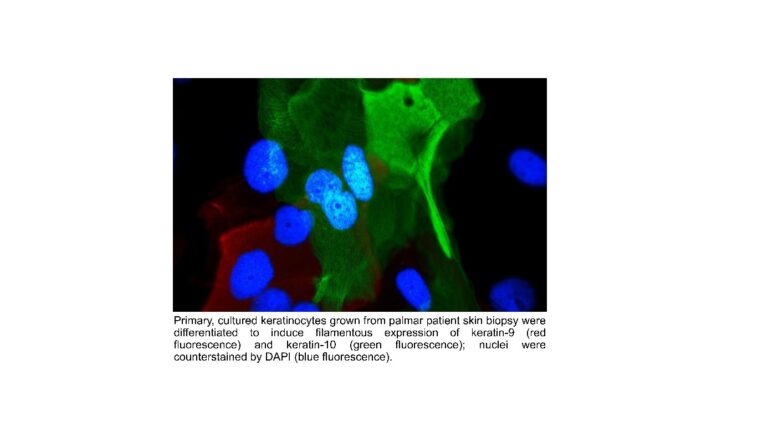
Epidermal homeostasis and genetic skin diseases
Matthias Schmuth, Daniela Ortner-Tobider, Susanne Tollinger, Thomas Trafoier, Susanne Kuipers, Robert Gruber.
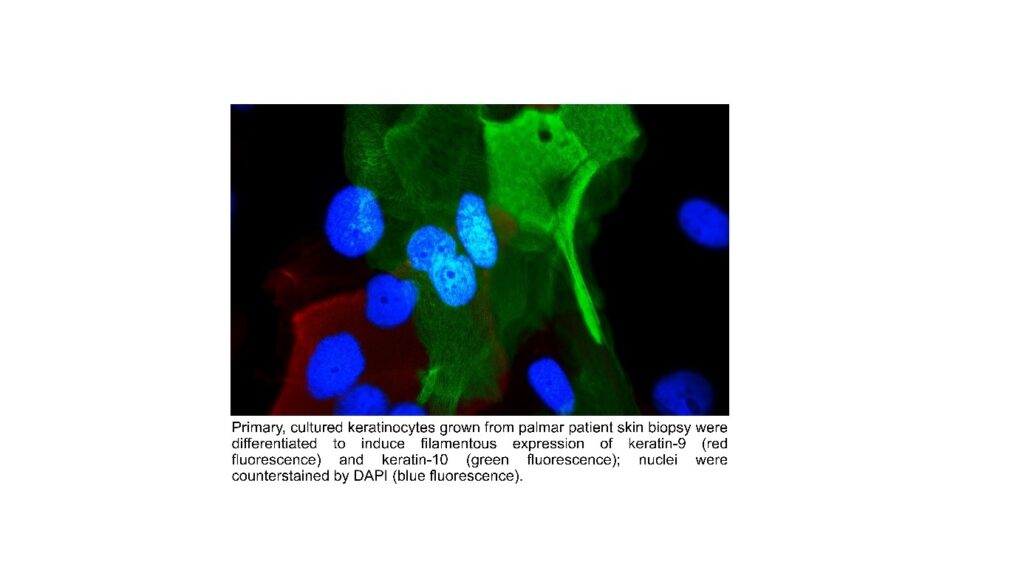
Our research focuses on the regulation of homeostasis and skin barrier function, as exemplified in rare epidermal differentiation disorders that result from defined gene mutations. The lab uses RNA sequencing, immunostaining of epidermal proteins, FACS analysis, LC-MS and MALDI imaging for the detection of lipids, biophysical skin measurements and various other techniques. The goals are to understand the pathogenesis of disease and to explore new treatments. A promising approach is correction of keratin filament fragility by means of gene editing (CRISP/Cas9, base editing). We have corrected the intermediate filament fragility in patient-derived keratinocyte cultures with keratin 9 mutations by means of CRISPR/Cas9-mediated deletion of the mutant, disease-causing allele while leaving the wild-type allele intact. The next step in translating our findings to the clinic will be delivering gene editing to skin cells in vivo. Other therapeutic approaches include small molecules and biologicals.
Cutaneous immune system, autoimmunity
Barbara Böckle, Magdalena Aichner, Gudrun Ratzinger
With the help of graduate students, this group is building a database of all patients with lupus erythematosus who have been treated at the clinic in the last 20 years. The database includes clinical and immune parameters along with information on response to therapy and will represent a rich scientific resource to address questions both of disease mechanisms and of therapeutic strategies. We are also participating in international networks working on bullous dermatosis, systemic sclerosis, pustular dermatosis and vasculitides.
Infectious diseases of the skin
Robert Zangerle, Mario Sarcletti, Martin Gisinger, Maria Kitchen-Hosp, Eva Meindl
This programme is addressing HIV epidemiology and response to therapy using the national AHIVCOS cohort, which is linked to international collaborative efforts, e.g. International Cohort Consortium of Infectious Disease (RESPOND), EuroSIDA, D:A:D Study, ART-CC, COHERE and PrEPARE.
Photomedicine
Gudrun Ratzinger
We are studying the effects of UV irradiation as a causative factor in photodermatoses and the therapeutic effects of UV irradiation on common inflammatory skin diseases and skin cancer. We are participating in the Austrian Psoriasis Registry (PsoRA), which includes gain clinical and epidemiological data. Collaborations with the Austrian Working Group of Photobiology offer insights and possibilities to participate in multicentre projects, e.g. to study vitiligo.
Dermatohistopathology
Nina Frischhut
The dermatohistopathological research in Innsbruck is renowned for the innovative concepts that have enabled a range of morphological discoveries in skin disorders.
Clinical Trials
Norbert Reider, Gudrun Ratzinger, Mario Sarcletti, Van Anh Nguyen, Georg Weinlich, Robert W. Gruber, Matthias Schmuth
The Department’s clinical trials unit is conducting numerous phase I – III clinical trials on chronic inflammatory skin disease (psoriasis), cutaneous and systemic lupus erythematosus, Sneddon’s Ssyndrome, bullous pempighoid/vulgaris, allergies, hidradenitis suppurativa, skin cancer (principally melanoma), HIV and genetic skin diseases.
Pictures
Selected Publications
- IL-36-driven pustulosis: Transcriptomic signatures match between generalized pustular psoriasis (GPP) and acute generalized exanthematous pustulosis (AGEP). Benezeder T, Bordag N, Woltsche J, Falkensteiner K, Graier T, Schadelbauer E, Cerroni L, Meyersburg D, Mateeva V, Reich A, Kołt-Kamińska M, Ratzinger G, Maul JT, Meier-Schiesser B, Navarini AA, Ceovic R, Prillinger K, Marovt M, Pavlovksy L, Szegedi A, Sanzharovskaja M, Zach H, Wolf P. J Allergy Clin Immunol. 2025: S0091-6749(25)00176-9. PMID: 39978684.
- Ortner D, Strandt H, Tripp CH, Spoeck S, Seretis A, Hornsteiner F, Dieckmann S, Schmuth M, Stoitzner P. Langerhans cells orchestrate apoptosis of DNA-damaged keratinocytes upon high-dose UVB skin exposure.Eur J Immuno 2024:e2451020. PMID: 39288297.
- Dudziak D, Heger L, Agace WW, Bakker J, de Gruijl TD, Dress RJ, Dutertre CA, Fenton TM, Fransen MF, Ginhoux F, Heyman O, Horev Y, Hornsteiner F, Kandiah V, Kles P, Lubin R, Mizraji G, Prokopi A, Saar O, Sopper S, Stoitzner P, Strandt H, Sykora MM, Toffoli EC, Tripp CH, van Pul K, van de Ven R, Wilensky A, Yona S, Zelle-Rieser C. Guidelines for preparation and flow cytometry analysis of human nonlymphoid tissue DC.Eur J Immunol, 2024: e2250325. PMID:
- Ortner-Tobider D, Trafoier T, Moosbrugger-Martinz V, Tollinger S, Gruber R, Schmuth M. Keratin variants in monilethrix. Br J Dermatol. 2024: 191:863-864. PMID: 39192774.
- Hornsteiner F, Vierthaler J, Strandt H, Resag A, Fu Z, Ausserhofer M, Tripp CH, Dieckmann S, Kanduth M, Farrand K, Bregar S, Nemati N, Hermann-Kleiter N, Seretis A, Morla S, Mullins DW, Finotello F, Trajanoski Z, Wollmann G, Ronchese F, Schmitz M, Hermans IF, Stoitzner P. Tumor-targeted therapy with BRAF-inhibitor recruits activated dendritic cells to promote tumor immunity in melanoma. J ImmunoTherap Cancer, 2024: e008606. PMID: 38631706.
- Schmuth M, Eckmann S, Moosbrugger-Martinz V, Ortner-Tobider D, Blunder S, Trafoier T, Gruber R, Elias PM. Skin Barrier in Atopic Dermatitis. J Invest Dermatol. 2024: 144:989-1000. PMID: 38643989.
- Minzaghi D, Pavel P, Kremslehner C, Gruber F, Oberreiter S, Hagenbuchner J, Del Frari B, Blunder S, Gruber R, Dubrac S. Excessive pProduction of hydrogen peroxide in mitochondria contributes to atopic dermatitis. J Invest Dermatol. 2023: 1906-1918.e8. PMID:
Selection of Funding
- Stand alone Project “Shine the spotlight on intratumoral DC2 and DC3 ” (2025-2029): PAT5895324 by FWF
- Stand alone project “Delivery of keratinocyte-targeting nano-carriers loaded with gene editing tools to intact skin” (2024-2027) by EI Cure
- MSCA doctoral Network “T-RAFIC:Tracking and controlling therapeutic immune cells in cancer” (2025-2029) by EU
- COST Action CA21108European Network for Skin Engineering and Modelling: NETSKINMODELS (2022-2026) by EU via COST-Action
- ESPRIT program “The role of NLRP3 inflammasome activation in Langerhans Cells for vitiligo” (2022-2025) by FWF
- Project “The role of the NLRP3 inflammasome in Langerhans Cells for skin homeostasis” (2022-2024) 29.000 € by TWF
- PhD-Graduate Program “Cellular Basis of Diseases” (2021-2025) by FWF
Collaborations
- Sulev Koks, Perron Institute, Australia
- Michele Boniotto, Institut Mondor for Biomedical Research/INSERM, Créteil, France
- Toni Cathomen, Institute for Transfusion Medicine and Gene Therapy, Medical Center-University of Freiburg, Germany
- Wei-Li Di, Institute of Cell and Molecular Science, Barts and the London School of Medicine and Dentistry, Queen Mary, University of London, UK
- Julian Valero Moreno and Prof. Jorgen Kjems, Aarhus University, Denmark
- Marc Schmitz and Dr. Rebekka Wehner, Technical University of Dresden, Germany
- Peter Elias, University of California, San Francisco, USA
- Michel Simon and Corinne Leprince, CNRS-INSERM-University of Toulouse, Toulouse, France
- Ivica Rubelj, Ruđer Bošković Institute, Zagreb, Croatia
- Christoph Messner, Precision Proteomics Center, Swiss Institute of Allergy and Asthma Research (SIAF), University of Zurich, Davos, Switzerland