Schöpfstraße 41
6020 Innsbruck
Email: Michaela.Kress@i-med.ac.at
Website: https://www.i-med.ac.at/dpmp/physiologie/
Research year
Research Branch (ÖSTAT Classification)
106006, 301110, 301407
Keywords
auditory physiology, epithelial physiology, erythrocyte, excitation-contraction coupling, iPSC derived human model systems, muscle, neuroimmune crosstalk, neuropathic pain, neuroregeneration, nociception, non-coding RNA, synapse formation, transcriptomic signatures, and Voltage-gated calcium channels
Research Focus
Research at the Institute of Physiology focuses on basic and preclinical translational studies on the (patho-)physiology of skeletal muscle, epithelial organs and the nervous system and employs an integrated, interdisciplinary approach to explore physiological processes in cellular models, organs and, recently, model systems derived from human iPSCs (induced pluripotent stem cells). Particular innovation potential arises from ongoing projects on sensory functions, calcium channels, nuclear kinases, the gut microbiome and non-coding RNAs.
General Facts
The institute is highly committed to medical research and a leading provider of human physiology education for undergraduate and graduate students at all levels. It is vital to understand healthy functions to explore the pathogenesis of common diseases, such as neurological and sensory disorders or chronic pain. The institute’s researchers engage in cutting-edge research on nociception, control of muscle contraction, auditory function, calcium signalling, cell membranes and epithelial physiology. We employ a wide range of models and techniques. These include cell culture, functional imaging, gene and protein expression, calcium microfluorimetry, high-resolution live microscopy and electrophysiology. We collaborate with local, national and international consortia. We are grateful for the funding provided by the European Commission, FWF, FFG and others. The FWF-Docfunds project CavX-2 is co-chaired by M. Campiglio; M. Kress is a beneficiary of MSCA-ITN TOBeATPAIN funded by the European Commission.
Devices & Services
Two-Photon Microscopy (Core facility Biooptics)
https://nociceptra.streamlit.app/Gene_Trajectories
https://biophysical-essentials.i-med.ac.at
Oxygen measurements (shared facility)
Research
Univ.-Prof. Dr. Bernhard E. Flucher – Calcium Channel Function
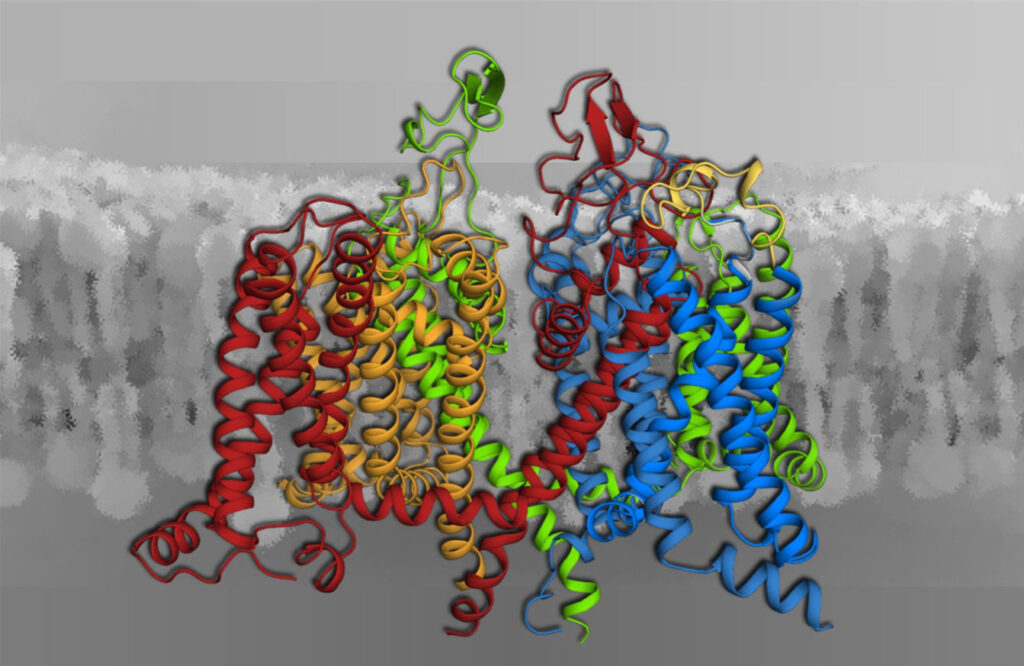
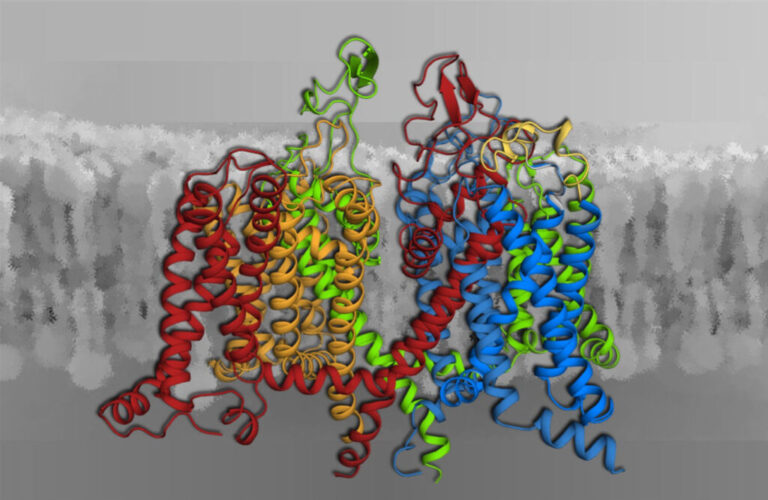
Voltage-gated calcium channels (Fig. 1) are the only proteins capable of sensing electrical signals at the cell surface and converting them into cell functions. These include the contraction of skeletal and heart muscle and the synaptic transmission in the nervous system. Our research covers the entire scope from exploring the biophysical mechanisms of channel functions at the molecular level to their roles in human physiology as well as nerve and muscle diseases. We apply cutting-edge genetic, molecular, biophysical, physiological and computational technologies.
Ao. Univ.-Prof. Dr. Johannes Fürst – Gastrointestinal Physiology
The mucosa of the intestines constitutes a selective barrier, which permits nutrient absorption and protection from undesired luminal content. At present, our focus is the function of the non-gastric H+/K+-ATPase ATP12A in regulating the colonic barrier and the role of selected neuropeptides and cytokines in the physiology of colonocytes.
Ao. Univ.-Prof. Mag. Dr. Thomas Haller – Respiratory Cell Physiology
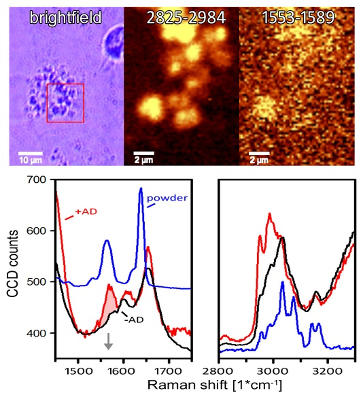
Reversible oxygen binding by haemoglobin is of fundamental importance in medicine and physiology. However, its routine determination has faced significant technical limitations in the past. We have developed a high-throughput plate reader-based approach to investigate O2 transport in the context of high altitude, accidental hypoxemia, relevant clinical interventions, pharmacological modulation and disease. Other ongoing studies are exploring surfactant and alveolar type II cells in the lung, as well as allosteric modulation of the oxygen affinity of haemoglobin in the microcirculation, which enhances the Bohr effect (Fig. 2).
Univ.-Prof. Dr. Michaela Kress – Neurobiology of Pain
Chronic pain disorders affecting approximately 20% of people represent a major socioeconomic burden on society, patients and their families worldwide. The Kress lab explores deregulated molecular signalling along the pain pathway. We are particularly focused on neuro-immune crosstalk involving cytokines, and non-coding RNA targeting transcriptional programmes. We are confident that we can bridge the translational gap between preclinical research and the lack of efficient therapies for patients. We have optimised iPSC-derived models to approximate native human functionalities with a comprehensive methodological approach. This exploits cutting-edge electrophysiology, molecular biology, transcriptomic profiling, complex bioinformatics and the development of novel software tools such as NOCICEPTRA or BPE.
Ass.-Prof. Dr. Kai Kummer – Pain Processing in Cortical Circuits
Our research focuses on plastic changes in the brain that give rise to the psychological comorbidities associated with chronic pain. We explore cholinergic projections from the basal forebrain to the medial prefrontal cortex as a promising pathway in the development of these comorbidities. We gain novel mechanistic insight through molecular tracing techniques, in vitro electrophysiological recordings, in vivo behavioural assays and opto- and chemo-genetic stimulations.
Ao Univ.-Prof. Dr. Judith Lechner – Impact of Didactic Methods on Learning Outcomes
Projects designed as prospective intervention studies analyse novel digital teaching and learning tools for their practicability and effectiveness to improve student learning outcomes in Medical Physiology. These projects focus especially on developing strategies to improve students’ and researchers’ awareness of animal-free methodology and the inclusion of sex and gender aspects.
Ass. Prof. Dr. phil. Christian Vogl – Auditory Physiology
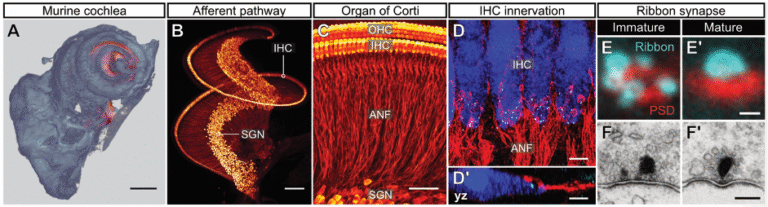
In the mammalian inner ear, synaptic sound encoding is achieved by ultrafast and tightly regulated neurotransmission between inner hair cell (IHC) ribbon synapses and postsynaptic spiral ganglion neurons (Fig. 3).
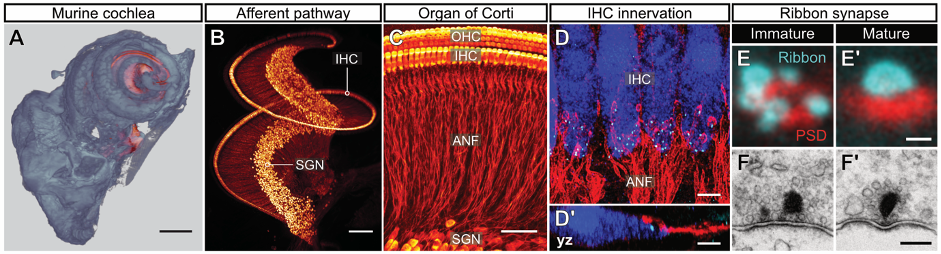
We combine fluorescence live-cell imaging of IHC synapses with immuno-histochemical analyses, super-resolution microscopy and optogenetic stimulation. This allows us to investigate cell biological processes underlying cochlear synaptogenesis. We are also identifying new deafness genes and developing gene-therapeutic treatments for hearing restoration.
Junior Research Groups
Dr. Marta Campiglio’s research is focused on unravelling the molecular mechanisms underlying skeletal muscle excitation-contraction coupling. She is specifically investigating the roles of various scaffold proteins, such as ERC1 and STAC3, in this process. Her work clarifies the genetic and molecular factors involved in the pathophysiology of congenital myopathies and malignant hyperthermia. Recently, Dr. Campiglio has expanded her collaborative research to explore the role of voltage-gated calcium channels (CaV) and their associated subunits in regulating cardiac pace-making.
Dr. Yousra El Ghaleb’s groundbreaking discovery identified CACNA1I, coding for T-type voltage-gated calcium channel CaV 3.3, as a disease gene for neurodevelopmental disease and epilepsy. She studies genotype-phenotype relations and the impact of disease variants on the structure and function of calcium channels. She uses electrophysiology, structure modelling, and neuronal computer models.
Dr. Theodora Kalpachidou explores the role of microRNAs (miRNAs) and their role in the complex responses to peripheral nerve injury related to neuropathic pain and neuronal regeneration. Several miRNAs are deregulated after a peripheral nerve injury and she aims to understand how these small RNA molecules regulate gene networks, how miRNA-controlled mechanisms promote neuro-regeneration and how they can be developed into novel therapeutic approaches.
Pictures
Selected Publications
Haller T, Jesacher A, Hidalgo A, Schmidt C. Life cell imaging of amiodarone sequestration into lamellar bodies of alveolar type II cells. Toxicol In Vitro. 2024. doi: 10.1016/j.tiv.2023.105733.
Pelizzari. S., Heiss, M.C., Fernández-Quintero, M.L., El Ghaleb, Y., Liedl, K.R., Tuluc, P., Campiglio, M., and Flucher, B.E. CaV1.1 voltage-sensing domain III exclusively controls skeletal muscle excitation-contraction coupling. Nature Communication, 2024, 15:7440; https://doi.org/10.1038/s41467-024-51809-5
El Ghaleb, Y. and Flucher, B. E. CaV3.3 channelopathies, Handbook of Experimental Pharmacology, 2023, pp. 1–26. 2023:279:263-288; https://doi.org/10.1007/164_2022_631
Voorn RA, Sternbach M, Jarysta A, Rankovic V, Tarchini B, Wolf F, Vogl C#. 2024. Slow kinesin-dependent microtubular transport facilitates ribbon synapse assembly in developing cochlear inner hair cells. eLife 13. doi:10.7554/eLife.98145.
Zimmermann D, Kress M, Zeidler M. Biophysical essentials – A full stack open-source software framework for conserved and advanced analysis of patch-clamp recordings. Comput Methods Programs Biomed. 2024 Oct;255:108328. doi: 10.1016/j.cmpb.2024.108328.
Zeidler M, Tavares-Ferreira D, Brougher J, Price TJ, Kress M. NOCICEPTRA2.0 – A comprehensive ncRNA atlas of human native and iPSC-derived sensory neurons.
iScience. 2023:26(12):108525. doi: 10.1016/j.isci.2023.108525.
Choconta JL, Labi V, Dumbraveanu C, Kalpachidou T, Kummer KK, Kress M. Age-related neuroimmune signatures in dorsal root ganglia of a Fabry disease mouse model.
Immun Ageing. 2023:20(1):22. doi: 10.1186/s12979-023-00346-8.
Patents
EP 4237825 A1, US 20230408474 A1
Selection of Funding
Stand-alone FWF projects:
P33776: Analysis of the functions of multiple STAC interactions (Campiglio)
PAT8455323: Mechanisms of enhancing skeletal muscle EC coupling by ERC1 (Campiglio)
ESP461: Structural basis of CaV3.3 calcium channel gating mechanisms (El Ghaleb)
P35618: Regulation of Ca currents and EC coupling by CaV1.1 voltage sensors (Flucher)
P33270: The role of CaV1.1 calcium channels in presynaptic differentiation during neuromuscular junction development (Flucher)
FWF, P36229: GAPmir: A novel miRNA in the GAP43 gene regulating neuroregeneration (Kalpachidou)
P344030: POCDMiBIOME (Kress)
PAT2555923: Maturational refinement of cochlear ribbon synapses (Vogl)
FWF DocFunds:
DOC-178: PhD program: Calcium channels in excitable cells (PIs: Campiglio, Vogl)
International:
PIN5091724: STAC3 disorder: gene therapy and malignant hyperthermia (Campiglio, PI)
I050870: PainMSK (Kress, PI)
European Commission, MSCA-ITN “TOBeATPAIN” GA764860 (Kress, PI)
FWF, I5364: LIAISON: Neuartige Interaktion von Kv Kanälen (Kummer, PI)
PIN5995324: HairSpike – how pH controls the spiking pattern in developing auditory sensory
cells (Vogl, PI)
Collaborations
Monica L. Fernández-Quintero, The Scripps Research Institute, La Jolla, CA, USA
Kerstin Kutsche, University Medical Center Hamburg-Eppendorf, Hamburg, Germany
Klaus Liedl, University of Innsbruck, Austria
Jocelyn LaPorte, Illkirchen, France
Matteo Mangoni, CNRS, Montpellier, France
Marzia Malcangio, King’s College London, United Kingdom
Nadine Ortner, University of Innsbruck, Austria
Filip Van Petegem, University of British Columbia, Canada
Theodore J. Price, University Dallas, USA
Hermona Soreq, Hebrew University Jerusalem, Israel
Jörg Striessnig, University of Innsbruck, Austria
Filip Van Petegem, University of British Columbia, Canada