Peter-Mayr-Straße 1/1a
6020 Innsbruck
Fax: +43 (0)512 9003 73200
Email: Hans.G.Knaus@i-med.ac.at
Website: https://www.i-med.ac.at/pharmakologie/
Research year
Research Branch (ÖSTAT Classification)
301206
Keywords
anxiety disorders, autoimmunity, calcium signalling, Epileptogenesis, Fear learning, G-protein coupled receptor, Gene therapy, Interneurons, ion channels, Metabotropic glutamate receptors, Neuropeptides, Opioid receptors, sleep research, and toll-like receptors
Research Focus
- Characterisation of the neural networks underlying physiological and pathological fear/anxiety and identification of novel treatment strategies
- Interaction of hunger and fear with a specific focus on neuropeptide systems
- Aetiology and novel treatment of temporal lobe epilepsy
- Disease specific engineering of designer receptor gene therapies
- Research on Toll-like receptors
General Facts
- The Department of Pharmacology, is a leading centre for research in neuro- and psychopharmacology, with a rich history dating back to 1886. Its approach is cutting-edge. It employs a variety of experimental methods to address fundamental research questions. These questions are related to the identification of novel molecular targets and the development of new therapeutic concepts for neuropsychiatric disorders.
- The department is responsible for providing pharmacology training to medical undergraduate and graduate students. The institute is committed to promote pharmacology and neuroscience within national and international societies. The Department of Pharmacology is a leading provider of independent drug and therapeutic information to doctors and contributes to a variety of public bodies (e.g. the EMA) involved in the evaluation of drug safety and development.
Research
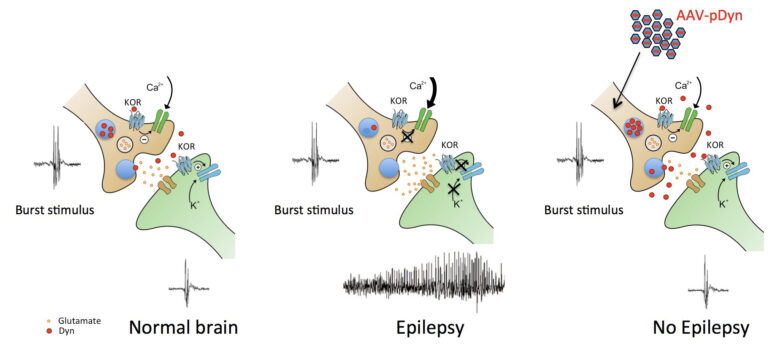
The kappa opioid system as drug target for temporal lobe epilepsy
Univ.-Prof. Mag.rer.nat. Dr. Christoph Schwarzer
The laboratory is investigating novel treatment options for temporal lobe epilepsy. These are based on a deeper understanding of the role of the endogenous dynorphin/kappa opioid receptor (KOPr) system in epilepsy and epileptogenesis. It also aims to gain further insight into the functional neuroanatomy of the dynorphin/KOPr in emotional control.
Epilepsy is a common, currently incurable neurological disease. Pharmacological treatment does not stop seizures in more than 30% of patients. Surgical removal of parts of the brain is the only solution, but restricted to a small group of patients. We are investigating the role of the endogenous dynorphin / kappa opioid receptor system in epilepsy and epileptogenesis as part of our work to find new treatment options. Our recent studies proofed that viral vector-derived preprodynorphin over-expressions in the epileptogenic focus suppress seizures over time. Gene therapy restored learning abilities in the kainic acid model of temporal lobe epilepsy. We are currently investigating the spread of gene therapy in the brain in order to understand the risks of side effects. This is done based on studies related to the functional neuroanatomy of the dynorphin / kappa opioid receptor system.
Our second line of investigation is focused on the role of paroxysmal depolarisation shifts in epileptogenesis in collaboration with H. Kubista, Med. Uni. Vienna.
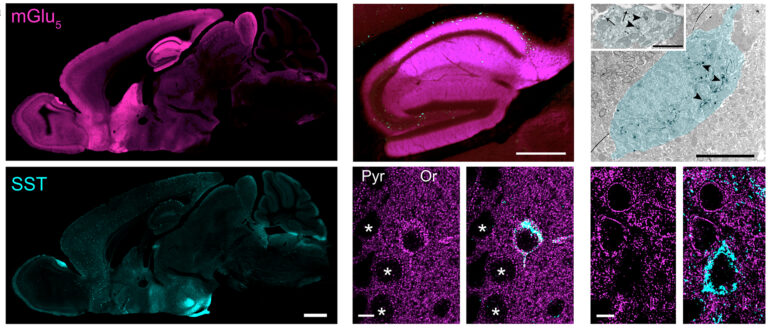
Neural circuits underlying emotional behaviour
Univ.-Prof. Dr. Francesco Ferraguti
The laboratory’s primary focus is on deciphering the brain’s processing of emotional information, with a particular emphasis on the pivotal roles of classical neurotransmitters (e.g. glutamate and GABA) and their receptors in anxiety and other negative emotions.
We have recently demonstrated that metabotropic glutamate 5 (mGlu5) receptors significantly contribute to the excitability of cortical somatostatin-expressing interneurons, which in turn regulate negative emotional states. We have also defined the behavioural function of an engram formed exclusively by GABAergic inhibitory neurons in the central amygdala. This engram disinhibits the activity of downstream areas to selectively control the expression of fear.
Our recent work focuses on how vasoactive intestinal peptide (VIP)-expressing interneurons (IN) in the anterior insular cortex of rodents gate sensory inputs and salience detection to adaptively shape behaviour. We hypothesise that their atypical function leads to impairments that manifest as anxiety and ASD core symptoms. Another key aim is to identify the neural circuits through which the pharmacological antagonism of mGlu5 receptor exerts its anxiolytic activity.
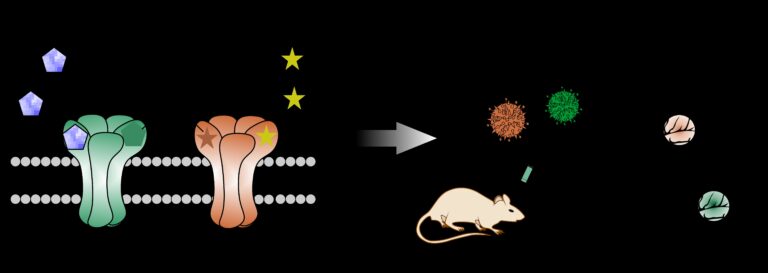
Disease specific engineering of designer receptor gene therapies
Univ.-Prof. Andreas Lieb PhD
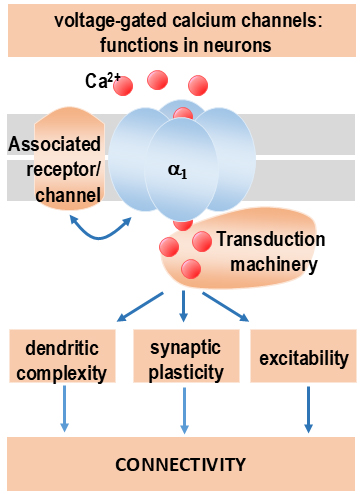
Our laboratory’s primary research focus is the preclinical development and optimisation of gene therapies for neurological disorders. These include epilepsy, Parkinson’s disease, neuropathic pain, and multiple sclerosis. Two prevalent conditions are refractory epilepsy and Parkinson’s disease, affecting over 21 million and 6 million patients worldwide, respectively. Despite extensive research and significant advancements in treatment strategies, both conditions remain challenging to treat.
Multiple gene therapy approaches targeting Parkinson’s disease and refractory epilepsy have been reported. However, existing strategies are limited by several drawbacks, which we aim to overcome by disease-specific engineering of designer receptors.
In recent years, we have developed a biochemically autoregulatory gene therapy designed to target refractory epilepsy. This system is activated only in response to pathological increases in the excitatory neurotransmitter glutamate. It thereby suppresses seizure activity in a controlled manner. Additionally, in collaboration with our research partners, we have designed a novel receptor that can be activated only by external application of a non-prescription agent, representing a significant step toward clinical translation. (Fig. 3)
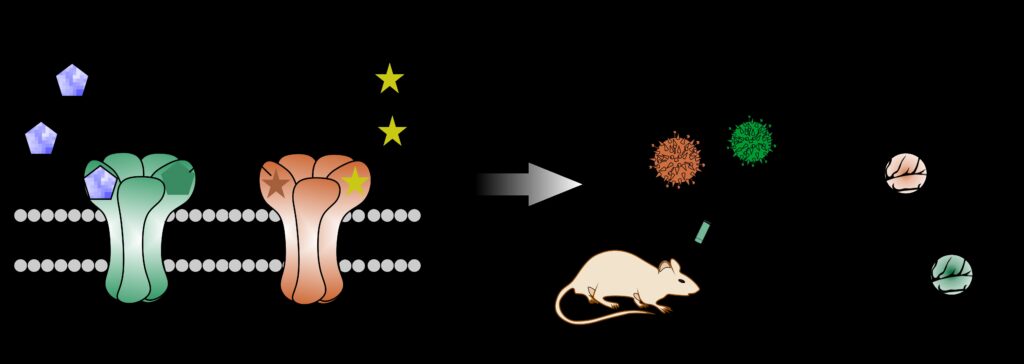
These technologies mark important progress, but they still face certain limitations. However, they clearly demonstrate the feasibility and potential of custom-engineered, disease-specific designer receptors, bringing us closer to more precise and effective gene therapies for neurological disorders.
Neuropeptides at the Crossroads of Fear and Hunger
Assoz. Prof. Dr.med.univ. Ramon Osman Tasan PhD
Avoiding danger and finding food are two intimately connected, life-sustaining phenomena. These are organised in survival circuits and strongly modulated by emotions. Maladaptation within such circuits can induce dysregulated behaviour, which undoubtedly results in the development of feeding and anxiety disorders. Neuropeptides are vital modulators of energy homeostasis and anxiety-related behaviours.
The laboratory investigates the role of neuropeptide transmitters in emotional behaviours related to fear and hunger. These neuropeptides are highly enriched in the extended amygdala, the brain area that controls emotional responses.
Our multidisciplinary approach, has demonstrated that several neuropeptides of the gut-brain axis are fundamentally involved in the modulation of fear and anxiety. This effect is highly dependent on the homeostatic situation of an individual. Neuropeptide Y, for example, is released during states of hunger and has potent anxiolytic and fear-reducing properties. Conversely, neurokinin B, an anxiogenic neuropeptide, reduces food intake. This places significant emphasis on the neuropeptide-dependent mutual interaction of survival circuits for fear and hunger.
Priv.-Doz. Mag.rer.nat. Meinrad Drexel PhD
The Drexel lab investigates the contribution of GABAergic interneurons and inflammation to temporal lobe epilepsy and epilepsy-related changes in sleep patterns.
Research Topic 1: The Role of Hippocampal Interneurons in Epileptogenesis
In temporal lobe epilepsy, seizures often originate in the hippocampus, where GABAergic interneurons regulate excitatory activity. We use transgenic mice with targeted expression of tetanus toxin light chain (TeLC) to specifically impair neurotransmitter release from subgroups of GABAergic interneurons. This allows us to study the impact of specific interneuron subtypes on epileptogenesis. Our research demonstrates that silencing GABA/parvalbumin and GABA/somatostatin neurons leads to spontaneous seizures, while GABA/VIP neurons do not. Future studies will explore additional GABAergic subtypes to gain a more complete understanding of their roles in epilepsy.
Research Topic 2: Neuroinflammation in Epilepsy
Neuroinflammation is a key factor in epilepsy, contributing to epileptogenesis and spontaneous seizures. Our group studies the relationship between epilepsy, neuroinflammation and the innate immune system. We are focused on toll-like receptors (TLRs), with an emphasis on TLR2. We found that TLR2 expression increases in glial cells and neurons after status epilepticus and that inhibiting TLR2 signalling reduces seizure severity. Conversely, activating TLR2 before seizure induction worsens outcomes. These findings highlight TLR2’s role in epilepsy and its potential as a target for novel antiepileptic therapies.
Research Topic 3: Epilepsy-Related Changes in Sleep Patterns
The hippocampal formation is a structure damaged in temporal lobe epilepsy (TLE) and projects to all major sleep-wake regulatory regions. Sleep studies show that TLE patients experience shortened, fragmented sleep, increased non-rapid eye movement (NREM) stage 1 sleep, and reduced rapid eye movement (REM) sleep, along with altered oscillatory activity. Seizure frequency does not correlate with the degree of sleep disturbance. This means that sleep disruption in epilepsy can result from independent circuit-level dysfunctions rather than being solely a consequence of seizures. In a pilot study with the intrahippocampal kainate epilepsy model, we investigated sleep macro- and microstructure in epileptic and control mice. We found significant epilepsy-associated sleep alterations and will explore the bidirectional relationship between sleep disturbances and epilepsy in future research.
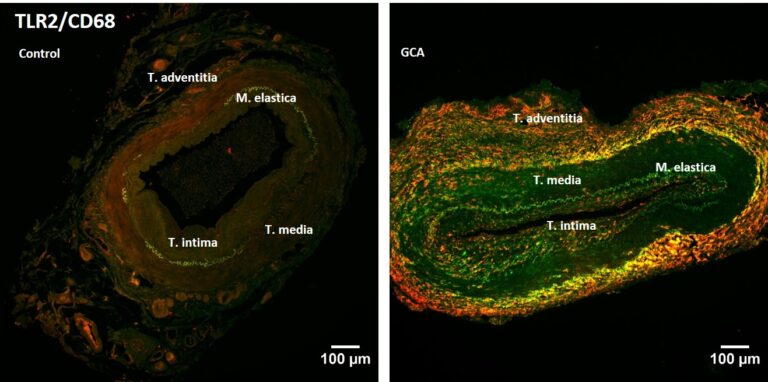
Priv.-Doz. Dr.rer.nat Sandra Santos Sierra
We are determined to understand the molecular mechanisms underlying the innate immune response in inflammatory diseases. Based on this knowledge, we will develop novel therapies (e.g. small molecules, peptides) that can be applied specifically to modulate immune activity. We are currently developing new alternative therapies for inflammatory and autoimmune diseases, including giant cell arteritis, polymyalgia rheumatic or systemic lupus erythematosus.
Topic 1:
TLRs are being explored as targets in a variety of inflammatory diseases. Toll-like receptor 2 (TLR2) recognises bacterially derived di- and tri-acylated lipopeptides and it also initiates an inflammatory response following recognition of endogenous host ligands produced during cellular stress and tissue damage (e.g. HMGB1, hyaluronan). We have discovered various compounds, synthetic small-molecules (Murgueitio, MS. et al. 2014 and 2017) and peptides (Ebner, S. et al. 2018) that modulate TLR2 receptor activity and other inflammatory pathways (e.g. IL-1R and TNF-R; Drexel, M. et al. 2019). We are currently testing the efficacy of the inhibitory compounds in several relevant disease models (Wietzorrek, G. et al. 2019). For example, in Polymyalgia rheumatica and giant cell arteritis (Seidlberger, S. et al 2025), the expression of TLRs plays a major role (Fig. 4).
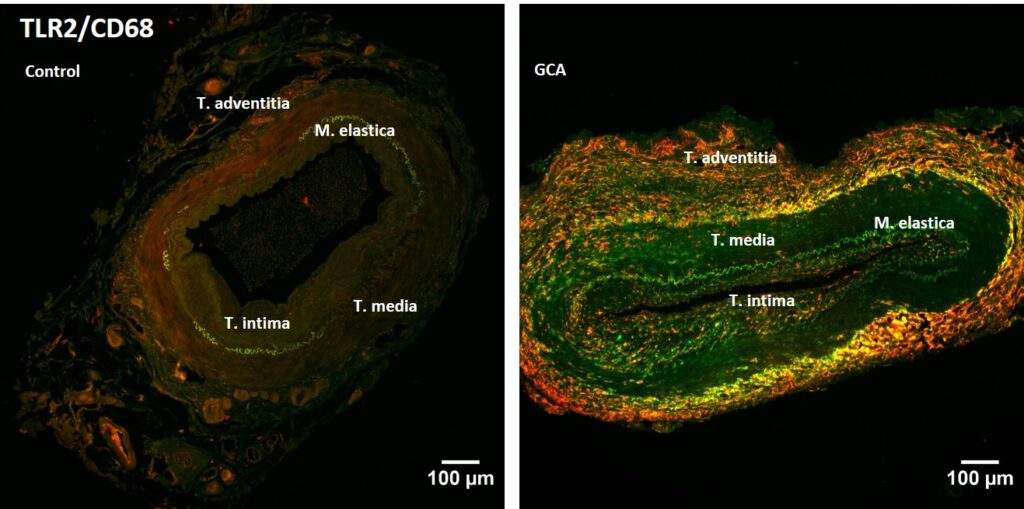
The standard therapy is glucocorticoid treatment, which is accompanied by marked secondary effects. New therapies are therefore needed.
Topic 2:
TLRs are expressed in immune cells, microglia and neurons in the central nervous system. However, the function of neuronal TLRs has only recently been uncovered. We have conclusively demonstrated the role of TLR8 in the development of temporal lobe epilepsy (Seizer, L. PLoS One, 2022). We are now moving on to investigate the function of neuronal TLR2 and its cognate partners TLR1 and TLR6 (Fig. 5)
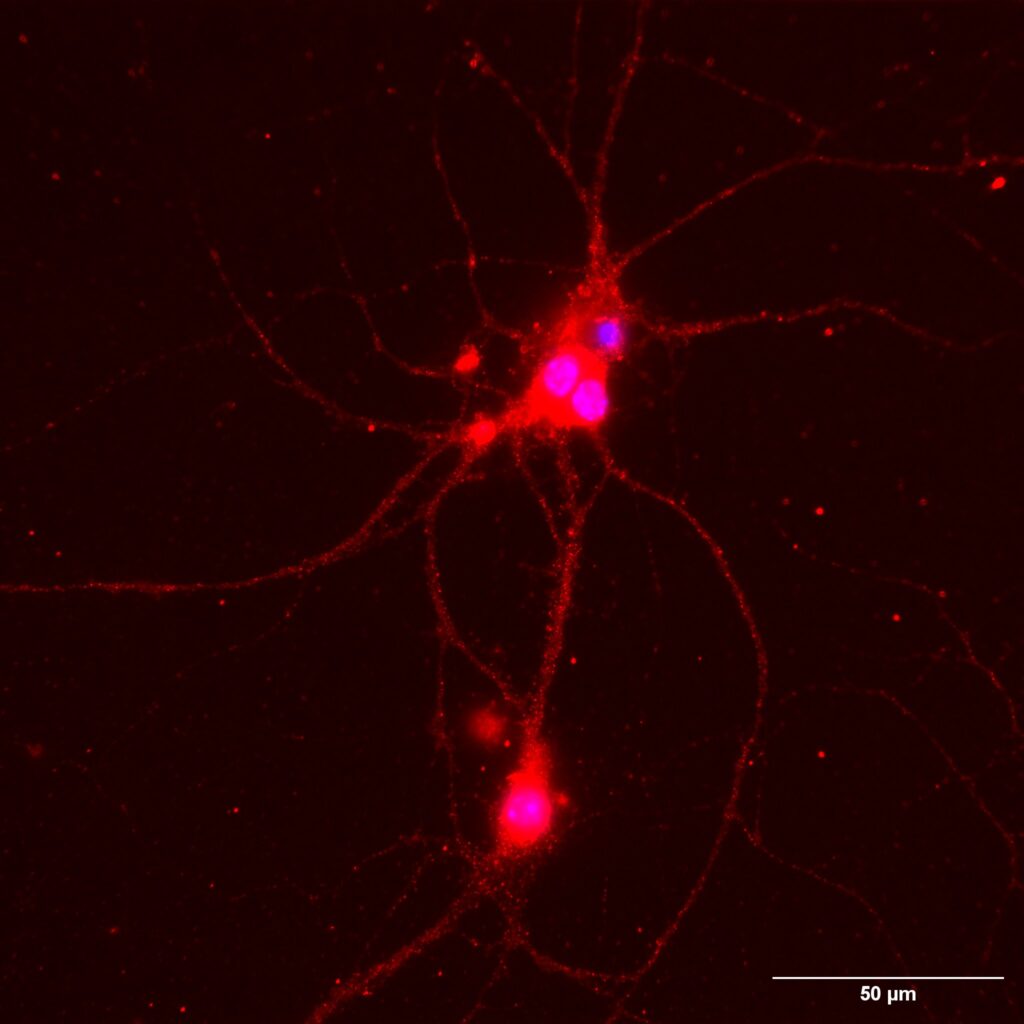
and their contribution to the origin and development of neurodegenerative diseases.
Mag.pharm. Valentina Di Biase PhD
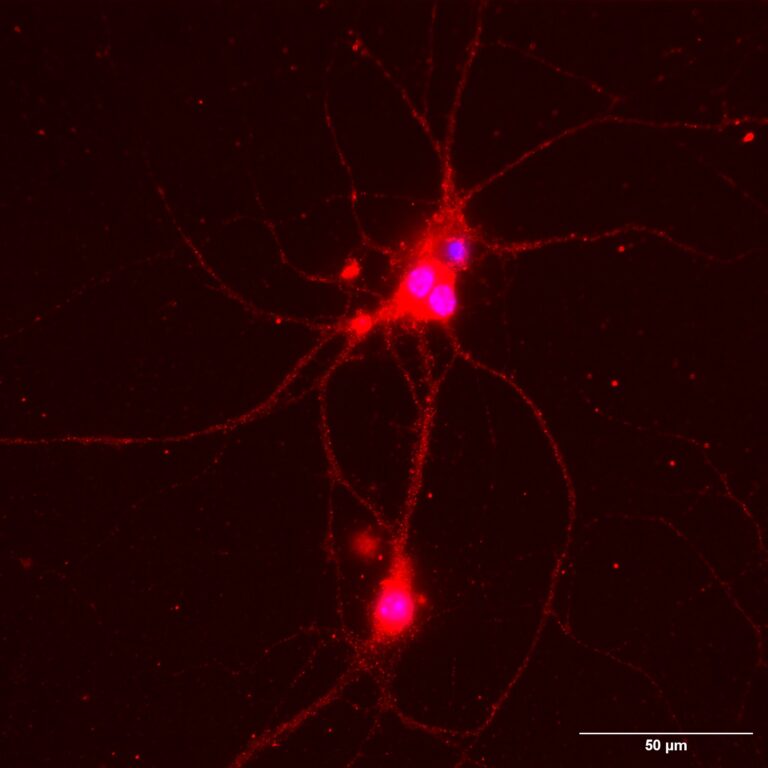
The Di Biase Lab focuses on research into ion channel regulation in health and diseases. This laboratory studies the mechanisms underlying neuronal structural and synaptic plasticity, membrane excitability, and signalling compartmentalisation in neurons and sinoatrial pacemaker cells.
The spectrum of interest is the regulation of ion channels in neurons, skeletal muscle and sinoatrial pacemaker cells. Our current research focuses on CACNA1C encoding high voltage gated calcium channels. This is a risk gene for psychiatric disorders and neurodevelopmental diseases linked to autism. Genomic studies are providing growing evidence of the enormous impact of these channels in brain conditions. However, the lack of information on their specific functions and cellular effectors prevents from identifying and implementing effective treatments. We are studying the channel functions underlying abnormal neuronal arborisation and synaptogenesis. This ultimately leads to disruption of normal brain circuitry and connectivity. We also explore the channel domains underpinning the pacemaker function in excitable cells. Our research is focused on identifying precise mechanisms as targets for developing novel and tailored pharmacological strategies to comply with unmet medical needs.
The Institute Pharmacology participates in EU public health activities by sending Dr. Di Biase to the European Medicines Agency (EMA) in Amsterdam as the scientific national expert from Austria. The EMA secondment program is designed to facilitate the structure for a transfer of knowledge and expertise through institutions. Dr. Di Biase has gained expertise in the scientific requirements for preparing and assessing centralised clinical trials for human medicines. EMA competencies are definitely transferable to transitional research interests at MUI.
Ass.Prof. Dr.Georg Wietzorrek
Pharmacology of hearing loss: Extracellular vesicles (EVs) derived from the secretome of human mesenchymal stromal cells (MSC) contain numerous factors that exert anti-inflammatory effects. MSC-EVs are the promising cell-based therapeutics for the inner ear that will effectively attenuate inflammation-based side effects from cochlear implantation. This represents an unmet clinical need. Intracochlear EV application is safe and effective. This is being determined in a controlled clinical trial. G. Wietzorrek has pivotely contributed to the application of the first Phase I/II clinical trial.
Pictures
Selected Publications
Comeras LB, Hormer N, Mohan Bethuraj P, & Tasan RO (2021) NPY Released From GABA Neurons of the Dentate Gyrus Specially Reduces Contextual Fear Without Affecting Cued or Trace Fear. Front Synaptic Neurosci 13:635726.
Qi Y, et al. (2023) Agrp-negative arcuate NPY neurons drive feeding under positive energy balance via altering leptin responsiveness in POMC neurons. Cell Metab 35(6):979-995 e977.
Kugler, V., Lieb, A., Guerin, N., Donald, B. R., Stefan, E., & Kaserer, T. (2023). Disruptor: Computational identification of oncogenic mutants disrupting protein-protein and protein-DNA interactions. Commun Biol, 6(1), 720. https://doi.org/10.1038/s42003-023-05089-2
Lipopolysaccharide-induced sepsis impairs M2R-GIRK signaling in the mouse sinoatrial node.
Shrestha Niroj, Zorn-Pauly Klaus, Mesirca Pietro, Koyani N Chintan, Wölkart Gerald, Di Biase Valentina, Torre Eleonora, Lang Petra, Gorischek Astrid, Schreibmayer Wolfgang, Arnold Robert, Maechler Heinrich, Mayer Bernd, von Lewinski Dirk, Torrente G Angelo, Mangoni E Matteo, Pelzmann Brigitte, Scheruebel Suaanne. Proc Natl Acad Sci U S A. 2023 120(28):e2210152120.
Rahimi, Sadegh; Salami, Pariya; Matulewicz, Pawel; Schmuck, Armin; Bukovac, Anneliese; Ramos-Prats, Arnau; Tasan, Ramon Osman; Drexel, Meinrad: The role of subicular VIP-expressing interneurons on seizure dynamics in the intrahippocampal kainic acid model of temporal lobe epilepsy. EXPERIMENTAL NEUROLOGY. 2023; 370; 114580.
Niebrugge, N., Trovato, O., Praschberger, R., & Lieb, A. (2024). Disease-Associated Dopamine Receptor D2 Variants Exhibit Functional Consequences Depending on Different Heterotrimeric G-Protein Subunit Combinations. Biomedicines, 13(1). https://doi.org/10.3390/biomedicines13010046
Ramos-Prats A., Matulewicz P., Edenhofer M.L., Wang K-Y., Yeh C-W., Fajardo-Serrano A., Kress M., Kummer K., Lien C-C., Ferraguti F. (2024) Loss of mGlu5 receptors in somatostatin-expressing neurons alters negative emotional states. Mol Psychiatry 29:2774-2786. doi: 10.1038/s41380-024-02541-5.
Rahimi, Sadegh; Joyce, Leesa; Fenzl, Thomas; Drexel, Meinrad: Crosstalk between the subiculum and sleep-wake regulation: A review. JOURNAL OF SLEEP RESEARCH. 2024; e14134.
Rahimi, Sadegh; Soleymankhani, Amir; Joyce, Leesa; Matulewicz, Pawel; Kreuzer, Matthias; Fenzl, Thomas; Drexel, Meinrad: Discriminating rapid eye movement sleep from wakefulness by analyzing high frequencies from single-channel EEG recordings in mice. SCIENTIFIC REPORTS. 2023; 13(1); 9608.
Zangrandi L, Fogli B, Mutti A, Staritzbichler R, Most V, Hildebrand PW, Heilbronn R, *Schwarzer C.* Structure-function relationship of dynorphin B variants using naturally occurring amino acid substitutions. Front Pharmacol. 2024 Oct 30;15:1484730. doi: 10.3389/fphar.2024.1484730. eCollection 2024. PMID: 39539623 https://pubmed.ncbi.nlm.nih.gov/39539623/
Widmann M, Lieb A, Fogli B, Steck A, Mutti A, *Schwarzer C.* Characterization of the intrahippocampal kainic acid model in female mice with a special focus on seizure suppression by antiseizure
Emedications xp Neurol. 2024 Jun;376:114749. doi: 10.1016/j.expneurol.2024.114749. Epub 2024 Mar 11. PMID: 38467356 Free PMC article. https://pubmed.ncbi.nlm.nih.gov/38467356/
Aksoy-Aksel A., Ferraguti F., Holmes A. Lüthi A, Ehrlich I. (2025) Amygdala intercalated cells form an evolutionary conserved system orchestrating brain networks. Nature Neurosci 28(2):234-247. doi: 10.1038/s41593-024-01836-8.
Alice Santagostino, Stephanie Seidlberger, Armin Schmuck, Katharina Donat, Meinrad Drexel & Sandra Santos-Sierra1. Activation of Toll-like receptor 8 (TLR8) expressed in mouse hippocampal neurons modulates neuronal excitability. Submitted. J. Neuroinflammation. 2025.
Patents
C. Schwarzer:
PCT/EP2023/077251
Gene therapy vectors for the expression of preprodynorphin variants for
the treatment of epilepsy; EU/US/Ca/China/
Jp pending
Selection of Funding
Neurobiology of anxiety in autism spectrum disorders. FWF-FG18 B. (Ferraguti)
CoE “Neuronal Circuits in Health and Disease” FWF-COE 16-B (Ferraguti)
Epiblok Service Agreement D-151500-018-014 EpiBlock Therapeutics GmbH (Schwarzer)
Designer Rezeptor Gentherapie für die Parkinson’s Krankheit. FWF P33222 (Lieb)
Gezielte Gentherapie gegen Epilepsie. FWF P355790 (Lieb)
Bedeutung von NPY in Furcht und Essverhalten FWF P343200 (Tasan)
Neurokinin B in emotionalen und metabolischen Prozessen FWF P337270 (Tasan)
CoE “Neuronal Circuits in Health and Disease” FWF-COE 16-B (Tasan)
Modulation of inflammation in epilepsy. FWF P30779 (Drexel)
The role of hippocampal interneurons in epileptogenesis. FWF P31777 (Drexel)
Novel anti-inflammatory intervention in PMR and GCA, FWF P35219 (Santos-Sierra)
Dendritic complexity regulation by phospho-S1928 of CaV1.2, FWF P3322 (Di Biase)
Collaborations
- Regine Heilbronn, Charité – Universitätsmedizin Berlin
- Peter Hildebrand, Universität Leipzig
- Prof. Helmut Kubista Med. Uni. Wien
- Matthias Mann, MPI for Biochemistry, Martinsried
- Martin Holtkamp, Charité – Universitätsmedizin Berlin
- Carlos B. Duarte, University of Coimbra, Coimbra, Portugal
- Asla Pitkänen, University of Eastern Finland, Kuopio, Finland
- Thomas Fenzl, Ludwig-Maximilians-Universität München, München, Germany
- Assist. Prof. Kaserer T., Institute of Pharmacy – Pharmaceutical Chemistry, University of Innsbruck, Innsbruck, Austria
- Dr. Tawfeeq Shekh-Ahmad, School of Pharmacy, Faculty of Medicine, Hebrew University of Israel, Jerusalem, Israel
- Assist. Prof Janosch Heller, School of Biotechnology, Dublin City University, Dublin, Ireland
- Luthi Andreas, Friedrich Miescher Institute for Biomedical Research, Basel, Switzerland
- Novarino Gaia, ISTA, Klosterneuburg, Austria
- Dr. Herbert Herzog, Neuroscience Division, Garvan Institute of Medical Research, Sydney, New South Wales, Australia.
- Prof. Dr. Tibor Harkany, Department of Molecular Neurosciences, Center for Brain Research, Medical University of Vienna, Vienna, Austria.
- Santos Castañeda. Hospital Universitario de la Princesa, Madrid, Spain.
- Johannes Kirchmair: Computational Drug Discovery and Design Group. University of Vienna. Vienna. Austria.
- Matteo Elia Mangoni, Institute of Functional Genomics, Montpellier, France
- Department of Otorhinolaryngology, Head and Neck Surgery Hannover Medical School Hannover Germany, Research Program Nanovesicular Therapeutics Paracelsus Medical University (PMU) Salzburg Austria;
- Ethics committee of Innsbruck Medical University, Ethics committee of the county of Salzburg