Research year
Research Branch (ÖSTAT Classification)
301402,106023,106051,106002
Keywords
axon guidance, axon regeneration, decision-making, Developmental neurobiology, in-vivo electrophysiology, prefrontal cortex, stroke models, system Neuroscience, and translational psychiatry
Research Focus
The Institute is home to four independent research groups, whose scientific interests range from investigating molecular, cellular and biochemical aspects of axon development and regeneration to studying basic processes or potential therapeutic interventions of ischemic stroke. Since December 2020, the Institute is also host to a new group focusing on translational models of psychiatric diseases and cognitive processes. The aim of all research groups at the Institute is to transfer promising basic research data
General Facts
Academic staff members at the Institute of Neurobiochemistry are also vastly engaged in the teaching of human medicine, dental medicine, molecular medicine (bachelors’ and masters’) and PhD curricula. Christine Bandtlow also began her tenure as Vice President for Research and International Relations in 2013 and was re-elected by the University Council in 2017 and 2020.
The Institute runs a state-of-the art bio-imaging core facility, which provides access to instruments, technologies and services to researchers of the MUI and other research institutions.
Research
Molecular basis of axon guidance and target innervation (Bandtlow Lab)
Univ.-Prof. Dr. Christine Bandtlow
Giuseppe Vaccaro, PhD student
Dominik Brück, PhD student,
Florian Hofer, Diploma student
Maja Überegger, BMA
Research in the Bandtlow lab is focused on understanding how axonal guidance is orchestrated at the molecular level. The approach is based on combining molecular biology, cell biology, imaging and genetics to define the role of molecules in both orchestrating axon growth during the development and stabilisation of neuronal circuits during adult life. The lab studies primarily the role of Nogo receptors (NgRs) in the peripheral sensory nerves of mouse mutants as a model, but also performs some studies on chicken commissural spinal cord. Current projects in the lab include:
1) Nogo receptors as guidance molecules in the developing spinal cord
Nogo receptors (NgRs), particularly NgR1, were shown to inhibit axonal regeneration and synaptic plasticity in the adult central nervous system. In collaboration with Esther Stöckli´s lab in Zurich, we are now able to provide evidence for a physiological role of NgRs during the early in vivo development of chicken spinal cord. Both NgR1 and NgR3 are required for midline crossing and subsequent turning of post-crossing axons into the longitudinal axis of the spinal cord. NgR1, but not NgR3, forms a receptor complex with PlexinA2 during axon guidance. Overall, these findings provide a link between neural regenerative mechanisms and axon guidance developmental processes.
2) Nogo receptors during cochlea synapse function
Hearing relies on the precise synaptic transmission between cochlear inner hair cells and spiral ganglion neurons through afferent ribbon synapses. NgRs are involved in axon guidance and facilitate dendritic plasticity and synapse maturation in the brain, but they have not been studied in the cochlea. Interestingly, NgRs are primarily expressed by cochlear spiral ganglion neurons and localise to distinct ribbon synapses. NgR KO-mouse mutants have compromised hearing function.
Neuronal Checkpoints in Brain Development and Stroke Pathology (Baier-Bitterlich Lab)
Ao. Univ.- Prof. Dr. Gabriele Baier-Bitterlich
Stephanie zur Nedden, PhD, Post-Doc
Dido Weber, BMA
Motahareh Safari, PhD student
Carolin Garmsiri, Master Student
Magdalena Brunner, Master student
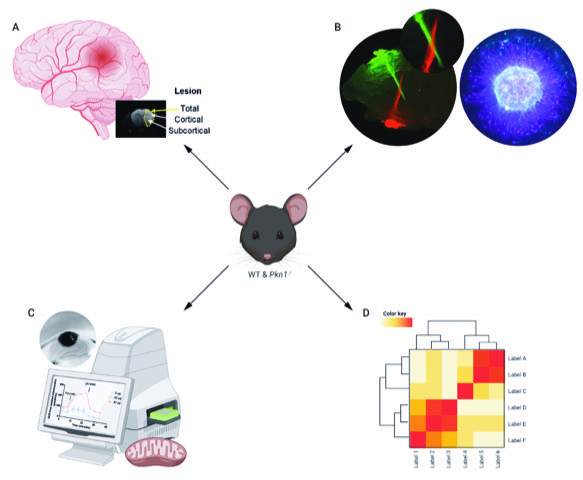
A central focus of our lab is to analyse the function of the serine/threonine kinase PKN1 in the central nervous system. Our research focuses on the development of the cerebellum, hippocampus and retina, all of which are areas with abundant PKN1 expressions. We further study the pathophysiological role of PKN1 in acute ischemia-reperfusion injury. For our research projects, we use a murine Pkn1 knockout model and employ biochemical, histological, molecular as well as metabolomics and transcriptomic analyses (Fig. 1).
1) Role of PKN1 in brain development
Recently, we have shown that PKN1 is a developmentally active regulator of cerebellar synaptic maturation by inhibiting AKT and the neurogenic transcription factor neurogenic differentiation factor-2 (NeuroD2). Pkn1 deletion results in hyper-activated AKT and increased NeuroD2 expression, thereby causing enhanced axonal outgrowth and presynaptic spacing of cerebellar granule cells. We were able to reveal a similar effect of Pkn1 deletion on AKT and NeuroD2 in the developing hippocampus, where it was accompanied by an increased expression of the NeuroD2 downstream target and AMPAR subunit GluA1 (Safari et al. 2021). Our data suggest that the tight control of postnatal AKT/NeuroD2 levels by PKN1 constitutes a general and important regulatory mechanism in the development of several brain areas and therefore validates PKN1 as a novel key player in neurodevelopment and neurodevelopmental disorders.
2) Preclinical evaluation of PKN1 as novel therapeutic target for ischemic stroke
Since the activation of the phosphoinositide-3-kinase/AKT cell survival-signalling pathway has become a promising new target for neuroprotective interventions, we are also studying the role of PKN1 during ischemia/reperfusion injury employing in vitro, ex vivo and in vivo models. Quite recently, we extended our field of interest to the detailed analysis of the regulation of gene expression and energy metabolism.
3) Role of PKN1 in retinal development and degeneration (Stephanie zur Nedden)
The main cause of incurable loss of vision lies in a permanent loss of retinal ganglion cells, the cells that give rise to the optic nerve and link the eye to the brain. A complete understanding of the developmental processes regulating the correct eye-brain connection is fundamental for the development of therapies that aim to restore vision. In the retina, both NeuroD2 overexpression and NeuroD2 knockout affect retinal cell type formation and stratification, demonstrating the importance of a tightly controlled balance of NeuroD2 protein levels for retinal development. In this project, we analyse the role of the novel PKN1-AKT-NeuroD2 axis in the development of the visual pathway as well as its role in acute stress-induced loss of retinal ganglion cells. In addition to developmental studies, we are investigating how the loss of PKN1 affects retinal energy metabolism and function.
Prefrontal neuronal circuits (PasseckerLab)
Assist. Prof. Johannes Passecker, PhD
Aron Koszeghy, PhD, Post-Doc
Sofia Castro e Almeida, PhD student
Alexander Wallerus, PhD student
Lukas Leitner, Diploma student
Leticia Wopfner, Diploma student
Our main research interest focuses on a better insight into how neuronal networks support cognitive control in both health and disease. How do we make decisions and how do the outcome and interpretation thereof affect our future goals, contextual interpretations and decisions? It is our aim to improve the neurobiological understanding of those processes and develop better model systems for stringent and efficient testing of potential rescue options of psychiatric disorders.
Together with colleagues and collaborators, we pursue an integrated approach by applying novel and state-of-the-art techniques to solve the scientific questions underlying psychiatric maladaptation of our brains. Our techniques involve head-fixed and freely moving in-vivo electrophysiology & behaviour, optogenetics, genetic model lines, RNA analysis, immunohistochemistry, behavioural testing combined with advanced statistical modelling, machine learning and deep-learning assisted analysis.
Cellular and molecular mechanisms of axonal regeneration in the PNS (Schweigreiter Lab)
Assoc. Prof. Dr. Rüdiger Schweigreiter
Dido Weber, BMA
Caren Agreiter, clinical PhD student
Walter Unterberger, Diploma student
Felix Schöffing, Diploma student
Ákos Kulimár, Diploma student
Hannah Sing, Diploma student
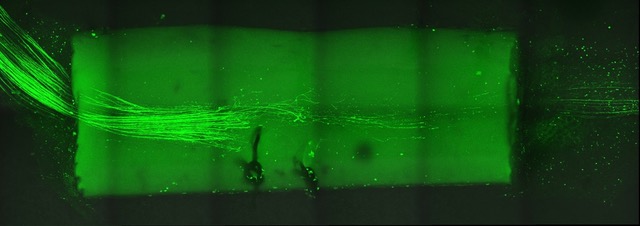
We are interested in cellular and molecular mechanisms of axonal regeneration in the adult peripheral nervous system with the aim to improve sensory and motor recovery after major nerve lesions. We work on identifying and characterising the molecular players that govern axonal outgrowth, long-distance growth and sprouting of nerve fibres after nerve lesion. To this end, we make use of a lesion model of the sciatic nerve in the mouse model, involving nerve transection and guidance of fibres through a synthetic nerve conduit. By applying advanced imaging and image processing techniques, we measure and quantify axon growth in vivo (Fig. 2&3).
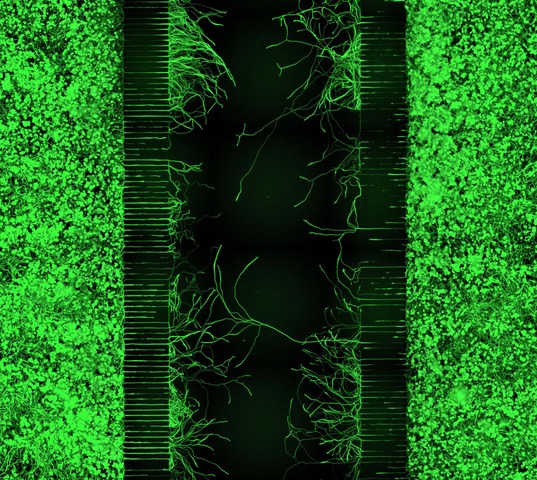
Moreover, we carry out in vitro experiments with primary sensory and motor neurons obtained from adult mice. We investigate the dynamics of axon growth in microfluidic chambers and analyse growth patterns by means of bio-orthogonal labelling.
Pictures
Selected Publications
Dassati S*, Schweigreiter R*, Buechner S, Waldner A. (2021) Celecoxib promotes survival and upregulates the expression of neuroprotective marker genes in two different in vitro models of Parkinson’s disease. Neuropharmacology 194:108378. doi: 10.1016/j.neuropharm.2020.108378
*…corresponding authors
Safari M.S., Obexer D., Baier-Bitterlich G., Zur Nedden S. PKN1 Is a Novel Regulator of Hippocampal GluA1 Levels. Front Synaptic Neurosci. 2021 Feb 5;13:640495. doi: 10.3389/fnsyn.2021.640495. eCollection 2021.
Vaccaro G., Dumoulin A., Zuñiga N. R., Bandtlow C.E. , Stoeckli E.T. The Nogo-66 receptors NgR1 and NgR3 are required for commissural axon pathfinding. Journal of Neuroscience 18 May 2022, 42 (20) 4087-4100; DOI: https://doi.org/10.1523/JNEUROSCI.1390-21.2022
Selection of Funding
Gabriele Baier-Bitterlich (P31085) FWF Stand-alone Research Grant 2018-2024, Title: Preclinical evaluation of PKN1 as stroke-target
Stephanie zur Nedden (T1091) Hertha Firnberg fellowship 2019-2024, Title: Role of PKN1 in retinal development and degeneration
Johannes Passecker – P 35747 FWF Stand-alone Research Grant 2022-2026, Title: Prefrontal-Striatal information routing for choice selection
Rüdiger Schweigreiter – P 33411 FWF Stand-alone research grant (2021-2024), Title: Improving axon regeneration in the peripheral nervous system
Collaborations
- Cyrille Orset, PhIND-INSERM/EFS/ Univ. Caen-Normandie, CAEN CEDEX, France
- Angus Cameron, Cancer Research Institute, London, UK
- Peter Parker, Francis Crick Institute, London, UK
- Esther Stöckli, Department of Molecular Life Sciences, Neuroscience Center Zurich, Zurich, Switzerland.
- Tobias Moser, Institute for Auditory Neuroscience and InnerEarLab, University Medical Center Göttingen, Göttingen, Germany.
- Joseph Gogos, Zuckerman Institute, Columbia University, New York, USA
- Joshua Gordon, National Institute of Mental Health, Bethesda, USA