Innrain 80-82
6020 Innsbruck
Fax: +43 (0)512 9003 73100
Email: Ludger.Hengst@i-med.ac.at
Website: https://www.i-med.ac.at/imcbc/medclinchemfolder/medclinchem.html
Research year
Research Branch (ÖSTAT Classification)
104002, 106002, 106037, 106052, 301114, 301211, 301303, 301904
Keywords
Cell Cycle Control, Cell Proliferation; Protein Kinases, Immunometabolism, Mass Spectrometry, proteomics, Toxicology, and Translational Control
Research Focus
The cell cycle group studies the molecular mechanisms that control the decision between cell proliferation and cell cycle arrest / exit into quiescence or terminal differentiation. The biochemical toxicology group investigates cellular responses to chemical exposure and the immuno-metabolic consequences. The regulatory science group aims to advance Non-Animal-Methodology for toxicology, focusing on developmental neurotoxicity, carcinogenicity and aquatic ecotoxicity.
General Facts
The institute hosts three research groups led by Ludger Hengst, Johanna Gostner and Martin Paparella as well as the protein core facility led by Bettina Sarg. The cell cycle and cell proliferation group uses a broad range of cell biological and molecular biological techniques to investigate the molecular mechanisms that govern the decision between cell proliferation and cell cycle exit to quiescence or differentiation in normal mammalian cells and in cancer cells. Our research focuses on the regulation of cyclin-dependent kinase (CDK) activities and CDK inhibitors and studies the mechanisms of transcriptional, translational and post-translational control. The Biochemical Immuno-Toxicology Group focuses on cellular responses to chemical exposure and its immuno-metabolic consequences. The Regulatory Science group is working to translate a new approach to toxicology based on non-animal methods into official chemical regulation. This niche has become important in reducing and replacing animal testing in toxicology. The protein core facility provides investigators with equipment, expertise and custom services for detecting, characterising and quantifying proteins and peptides. As well as LC-MS-based proteomic analyses, the facility offers services such as High-Performance Liquid Chromatography (HPLC), capillary electrophoresis, Edman degradation and elemental analysis using atomic absorption spectrometry.
Research
Cell Cycle and Cell Proliferation
Ludger Hengst
The growth, development and integrity of multicellular organisms depend on precisely coordinated cell division and differentiation. Cells are exposed to a variety of conflicting signals that aim to regulate cell growth, cell proliferation, differentiation or cell fate. These multiple internal and external signals act on the central cell cycle control machinery to either promote or block cell proliferation. These signals must be properly processed and integrated correctly to enable the decision between cell proliferation and quiescence and to maintain body and organ homeostasis. Incorrect interpretation, processing or integration of these signals can lead to hypo- or hyper-proliferative disorders, including diseases such as cancer and anaemia.
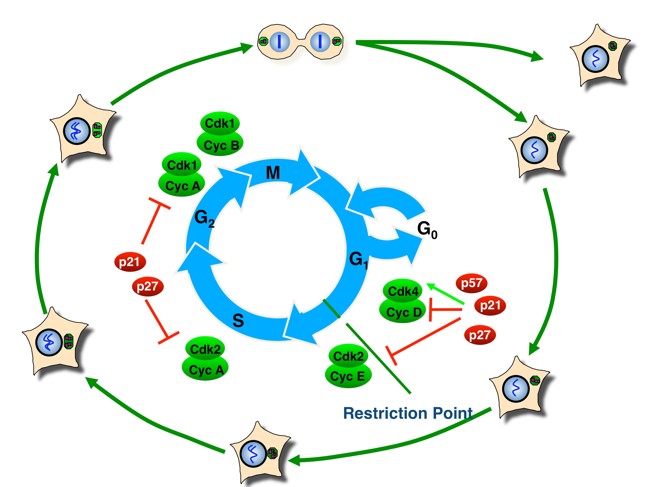
The decision to either proliferate or exit the cell cycle into quiescence is usually made at during a specific period of the eukaryotic cell division cycle. The cell cycle is subdivided into four phases: the G1 and G2 gap phases separate DNA replication (in S-phase) from the segregation of duplicated DNA and other cellular components (in M-phase, i.e. mitosis and cytokinesis). In G1 phase, cells decide to either withdraw from proliferation or to commit to another round of cell division. The decision to proliferate becomes irreversible once cells pass over the so-called restriction point in late G1 phase (Fig. 1). This renders cells mitogen-independent and committed to undergo another complete cell division cycle.
Our research focuses on investigating the molecular mechanisms that link diverse signalling networks to the central cell cycle control machinery. At the core of this machinery is a conserved family of protein kinases known as cyclin-dependent kinases (CDKs). CDKs are activated by binding to a positive regulatory subunit, called a cyclin. The sequential activation and inactivation of specific CDK complexes is required for cell cycle progression. CDK inhibitors, such as p21Cip1 (CDK-interacting protein), p27Kip1 (kinase-inhibitory protein) and p57Kip2 bind to CDKs and control their kinase activities. The expression, localisation and modification of these inhibitors play a central role in regulating progression through the G1-phase at the restriction point. In addition to their canonical function in CDK regulation, these proteins can also exert CDK-independent and / or cell cycle-independent functions. For example, the CDK inhibitor p27Kip1 has been shown to regulate cell motility and migration, linking this tumour suppressor protein to cancer metastasis. Interestingly, some non-canonical functions of Cip/Kip proteins, such as transcriptional control, can be CDK-dependent or CDK-independent.
Among others, we identified CDK inhibitor protein p27Kip1. The expression, activity, localisation and stability of this protein can be regulated by various mitogen signalling pathways. We are investigating how these pathways intersect to control the functions of the Cip/Kip family in normal and cancer cells.
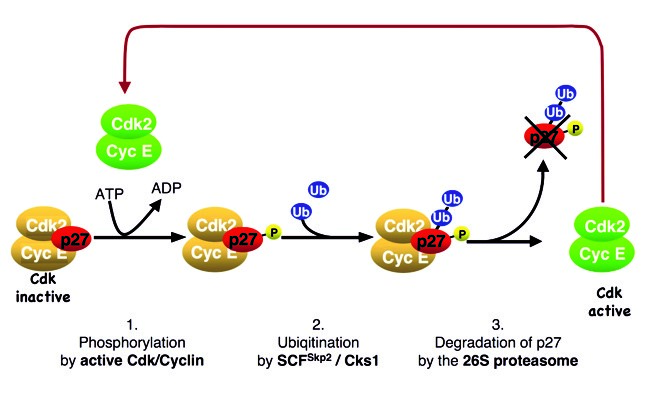
p27Kip1 regulates cell cycle progression at the restriction point. High levels of p27 can bind and inactivate CDKs, preventing cell proliferation. Following cyclin/CDK2 activation, the CDK inhibitor protein becomes unstable at about the time when cells traverse the restriction point and progress towards the S-phase. A positive feedback loop couples p27 ubiquitin-dependent degradation to CDK activation (Fig. 2). However, the molecular mechanism that can initiate this feedback loop in the presence of abundant CDK inhibitors and thus inactive CDKs has long remained an enigma.
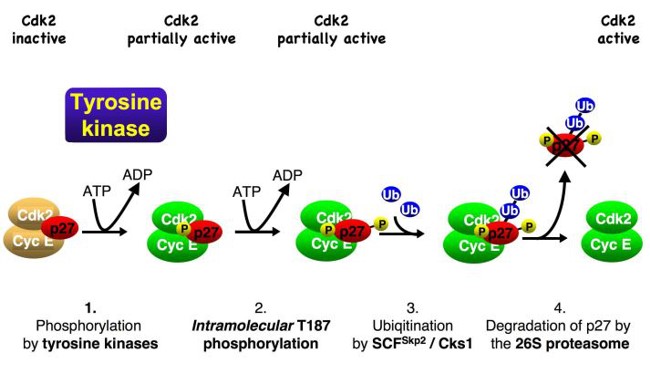
We observed that oncogenic tyrosine kinases, including BCR-Abl, Src, FLT3 and JAK2, can activate p27-bound CDK complexes by phosphorylating the inhibitor. Following this, an inhibitory helix of p27 is ejected from the catalytic cleft of the CDK, allowing the p27-bound CDK complex to bind ATP and phosphorylate substrates. One of these substrates is p27 itself. Phosphorylation of p27 by the bound CDK generates a phosphodegron, which can initiate SCFSkp2– mediated ubiquitin-proteasomal degradation of p27 (Fig. 3). Mitogen signals can inactivate and destabilise inhibitor p27 using this mechanism, which can be abused by oncogenic kinases, thereby promoting CDK activation and cell cycle progression.
Ongoing research projects
- Function and regulation of CDK-inhibitory proteins.
- Role of translational control in the choice between cell proliferation and withdrawal from the cell cycle.
- Cell cycle regulation by cytokine receptor signalling.
- Regulation of the SCFSkp2 Ubiquitin E3 ligase.
- Non-canonical functions of Cip/Kip proteins.
- Function of a conserved small peptide encoded in the uORF of the p27 mRNA.
Biochemical Immuno-toxicology
Johanna Gostner
Understanding biochemical and molecular processes is essential for deciphering how the human body responds to an ever-changing environment. Increasing exposure to environmental chemicals and emerging pathogens poses an increasing risk to human health, while nutrition, lifestyle and the microbiome composition further shape individual susceptibility.
Ongoing research
Our research combines analytical chemistry, biochemistry, cell biology and immunological approaches with modern biotechnological tools to explore how cells respond to the various substances that make up our exposome, such as airborne chemicals, ingredients in personal care products and naturally occurring phytochemicals. This will help us to gain a deeper understanding of the complex biological processes involved in sensitisation, irritation and cellular adaptation.
Our goals include validating in vitro testing methods and developing workflows for extrapolating in vitro findings to in vivo contexts. We also aim to investigate biomarkers in various disorders and occupational health settings. We are particularly interested in immuno-metabolism and amino acid and pteridine biochemistry, which are metabolic pathways influenced by drugs, chemicals, natural products, dietary components and physical activity. Several metabolites within these pathways serve as sensitive biomarkers of chronic inflammation, immune dysfunction and neuropsychiatric conditions, as well as being clinically relevant indicators of a range of disorders. They have promising potential for informing personalised treatment strategies.
Regulatory Science Group
This desktop-based work focuses on systematically retrieving toxicological data, qualifying it, innovatively integrating it, and characterising uncertainty for a chemical risk assessment based on non-animal methods. The work combines research projects with the activities of relevant OECD working groups.
In brief, the Regulatory Science Project, funded by the Ministry of the Environment, supports participation in European and OECD expert groups to promote the regulatory use of non-animal methods (NAMs). Projects such as ALTERNATIVE, DNT-RAP4, CHIASMA, INSIGHT and PARC, which are funded by various EU programmes, focus on developing NAMs for toxicological and environmental safety assessments. The ALTERNATIVE project uses computational and microphysiological models to address cardiotoxicity, while the DNT-RAP4 project, led by our team, develops AOP-based strategies for assessing pesticide-related developmental neurotoxicity, placing a strong emphasis on uncertainty characterisation. CHIASMA and INSIGHT are advancing a series of NAMs and computational methods for toxicology. They are also developing methodology to evaluate the impact of chemicals on carbon footprint, biodiversity and socioeconomics. Our role focuses on methodological developments and regulatory integration. Within PARC, we are spearheading NAM-based assessments for aquatic toxicity and genotoxicity, offering systematic analyses and robust uncertainty evaluations. We are also leading a proposal for the EPAA DESIGNATHON challenge, which aims to develop a new hazard classification system based entirely on non-animal-methods. We also chaired the discussion on the respective classification design principles.
Protein Core Facility
Bettina Sarg
Analysing proteins, from cells to entire organisms, provides insight into how biological processes work, because proteins are the agents of biological mechanisms. The challenge in proteomics is to identify, quantify and characterise proteins and peptides in complex systems.
The protein core facility supports proteomics-related projects, providing assistance with experimental design, data acquisition and bioinformatic analysis, as well as data interpretation. The facility’s infrastructure is centred around state-of-the-art mass spectrometry for the comprehensive identification of simple and complex protein digests from gel bands, immunoprecipitations and whole cell/tissue digests, as well as quantitative proteomics using isotope labelling strategies (e.g. SILAC and TMT). We are constantly developing and establishing novel analytical methods and technologies to advance proteomics and meet the users’ needs.
Moreover, we offer a comprehensive range of techniques for the separation of proteins and peptides, including High-Performance Liquid Chromatography (HPLC) and Capillary Electrophoresis (CE). We also provide N-terminal protein sequencing by Edman degradation and the quantitative determination of most elements (including all metals, metalloids and some nonmetals) in a wide variety of samples using atomic absorption spectrometry (AAS).
Pictures
Selected Publications
The Hengst Laboratory: Cell Cycle and Cell Proliferation Control
- EpoR Activation Stimulates Erythroid Precursor Proliferation by Inducing Phosphorylation of Tyrosine-88 of the CDK-Inhibitor p27Kip1. Pegka Fraga, Ben-Califa Nathalie, Neumann Drorit, Jäkel Heidelinde, Hengst Ludger. 2023 Jun 23;12(13):1704. doi: 10.3390/cells12131704. PMID: 37443738
- Inability to phosphorylate Y88 of p27Kip1 enforces reduced p27 protein levels and accelerates leukemia progression. Jäkel Heidelinde, Taschler Martin, Jung Karin, Weinl Christina, Pegka Fraga, Kullmann Michael Keith, Podmirseg Silvoio Roland, Dutta Sayantanee, Moser Markus, Hengst Ludger. Leukemia. 2022; 36(7):1916-1925. PMID: 35597806 doi: 10.1038/s41375-022-01598-x.
- The CDK inhibitor p57Kip2 enhances the activity of the transcriptional coactivator FHL2. Kullmann, Michael K.; Podmirseg, Silvio R.; Roilo, Martina; Hengst, Ludger, SCIENTIFIC REPORTS: 2020; April 28; 10 (1): 7140. doi:1038/s41598-020-62641-4.
The Gostner Laboratory: Biochemical Immunotoxicology
- Chemical respiratory sensitization-Current status of mechanistic understanding, knowledge gaps and possible identification methods of sensitizers. Hargitai R, Parráková L, Szatmári T, Monfort-Lanzas P, Galbiati V, Audouze K, Jornod F, Staal YCM, Burla S, Chary A, Gutleb AC, Lumniczky K, Vandebriel RJ, Gostner JM. Front Toxicol. 2024;6:1331803. doi: 10.3389/ftox.2024.1331803. eCollection 2024. PMID: 39135743
- Modeling omics dose-response at the pathway level with DoseRider. Monfort-Lanzas P, Gostner JM, Hackl H. Comput Struct Biotechnol J. 2025;27:1440-1448. doi: 10.1016/j.csbj.2025.04.004. eCollection 2025. PMID: 40242291
- Dietary digestible carbohydrates are associated with higher prevalence of asthma in humans and with aggravated lung allergic inflammation in mice. Musiol S, Harris CP, Karlina R, Gostner JM, Rathkolb B, Schnautz B, Schneider E, Mair L, Vergara EE, Flexeder C, Koletzko S, Bauer CP, Schikowski T, Berdel D, von Berg A, Herberth G, Rozman J, Hrabe de Angelis M, Standl M, Schmidt-Weber CB, Ussar S, Alessandrini F. Allergy. 2023;78(5):1218-1233. doi: 10.1111/all.15589. PMID: 36424672
- Toxoplasma gondii IgG Serointensity Is Positively Associated With Frailty. Mohyuddin H, Laffon B, Teixeira JP, Costa S, Teixeira-Gomes A, Pásaro E, Constantine N, Dagdag A, Ortmeyer HK, Tizenberg B, Afram L, Yen P, Marano C, Lowry CA, Hoisington AJ, RachBeisel JA, Valdiglesias V, Lema-Arranz C, Fernández-Bertólez N, Maseda A, Millán-Calenti JC, Kovacs EJ, Gostner JM, Fuchs D, Brenner LA, Lorenzo-López L, Postolache TT. J Gerontol A Biol Sci Med Sci. 2024;79(3):glad228. doi: 10.1093/gerona/glad228. PMID: 37939652
The Paparella Group: Regulatory Science
- Daphnids can safeguard the use of alternative bioassays to the acute fish toxicity test: A focus on neurotoxicity. Schür C, Paparella M, Faßbender C, Stoddart G, Baity Jesi M, Schirmer K. Environ Toxicol Chem. 2025;43(3):559–574. doi: 10.1093/etojnl/vgaf014. PMID: 39836637.
- INSIGHT: An integrated framework for safe and sustainable chemical and material assessment. Serra A, Zouraris D, Schaffert A, Torres Maia M, Tsiros P, Virmani I, Di Lieto E, Saarimäki LA, Morikka J, Riudavets-Puig R, Varsou D-D, Papavasileiou KD, Kolokathis PD, Mintis DG, Tzoupis H, Tsoumanis A, Melagraki G, Arvanitidis A, Doganis P, Minadakis V, Savvas G, Perello-Y-Bestard A, Cucurachi S, Buljan M, Nikiforou F, Karakoltzidis A, Karakitsios S, Sarigiannis DA, Friedrichs S, Seitz C, Gutierrez TN, Isigonis P, Cambier S, Marvuglia A, Lindner GG, Sergent J-A, Gheorghe LC, Bradford L-JA, Park S-G, Ha SM, Gerelkhuu Z, Yoon TH, Petry R, Martinez DST, Winkler DA, Wick P, Exner TE, Dondero F, Serchi T, Peijnenburg W, Sarimveis H, Paparella M, Lynch I, Afantitis A, Greco D. Comput Struct Biotechnol J. 2025;29:125–137. doi: 10.1016/j.csbj.2025.03.042. PMID: 40241814.
- A developmental neurotoxicity adverse outcome pathway (DNT-AOP) with voltage-gated sodium channel (VGSC) inhibition as a molecular initiating event (MiE). Crofton KM, Paparella M, Price A, Mangas I, Martino L, Terron A, Hernández‐Jerez AF. EFSA J. 2024;22(8):8954. doi: 10.2903/j.efsa.2024.8954.
- A decade of progress in the development of aquatic new approach methodologies from 2012 to 2022. Langan LM, Paparella M, Burden N, Constantine L, Margiotta-Casaluci L, Miller TH, Moe SJ, Owen SF, Schaffert A, Sikanen T. Environ Toxicol Chem. 2023;42(1):123–135. doi: 10.1002/etc.5578. PMID: 36722131.
- Cardiotoxicity of chemicals: Current regulatory guidelines, knowledge gaps and needs. Schaffert A, Murugadoss S, Mertens B, Paparella M. ALTEX. 2023;40(2):337–340. doi: 10.14573/altex.2301121. PMID: 36648099.
Bettina Sarg – The Protein Core Facility
- The Dsc ubiquitin ligase complex identifies transmembrane degrons to degrade orphaned proteins at the Golgi. Weyer Y, Schwabl SI, Tang X, Purwar A, Siegmann K, Ruepp A, Dunzendorfer-Matt T, Widerin MA, Niedrist V, Mutsters NJM, Tettamanti MG, Weys S, Sarg B, Kremser L, Liedl KR, Schmidt O, Teis D.: Nat Commun. 2024 Oct 26;15(1):9257. doi: 10.1038/s41467-024-53676-6. PMID: 39461958
- Silicone implant surface microtopography modulates inflammation and tissue repair in capsular fibrosis. Schoberleitner I, Faserl K, Tripp CH, Pechriggl EJ, Sigl S, Brunner A, Zelger B, Hermann-Kleiter N, Baier L, Steinkellner T, Sarg B, Egle D, Brunner C, Wolfram D.: Front Immunol. 2024 Mar 19;15:1342895. doi:10.3389/fimmu. 2024.1342895. PMID: 38566997.
- Truncated variants of MAGEL2 are involved in the etiologies of the Schaaf-Yang and Prader-Willi syndromes. Heimdörfer D, Vorleuter A, Eschlböck A, Spathopoulou A, Suarez-Cubero M, Farhan H, Reiterer V, Spanjaard M, Schaaf CP, Huber LA, Kremser L, Sarg B, Edenhofer F, Geley S, de Araujo MEG, Huettenhofer A.: Am J Hum Genet. 2024 Jul 11;111(7):1383-1404. doi: 10.1016/j.ajhg.2024.05.023. Epub 2024 Jun 21. PMID: 38908375
- PKN1 Exerts Neurodegenerative Effects in an In Vitro Model of Cerebellar Hypoxic-Ischemic Encephalopathy via Inhibition of AKT/GSK3β Signaling. Zur Nedden S, Safari MS, Fresser F, Faserl K, Lindner H, Sarg B, Baier G, Baier-Bitterlich G. Biomolecules. 2023 Oct 31;13(11):1599. doi: 10.3390/biom13111599.
Selection of Funding
- FWF: WEAVE, I 6122: UVISION 1.1 Grant-DOI 10.55776/I6122
- FWF TAI 432 1000 Ideen Programm: Neue Aufgaben für kleine uORF-kodierte Peptide. Grant-DOI 10.55776/TAI432
- FWF: Doc 82/10 : Cellular Basis of Diseases: Molecular Control of Metabolism and Inflammation; Faculty Grant-DOI 10.55776/DOC82
- HPCE method development for the determination of size and loading efficiency of nanoparticles. Industrial Project with RNAnalytics
- FWF: Forschergruppe 2006–B: Partner: TSC-Regulatoren steuern ER-Lysosomen-Crosstalk und Wachstum. Grant-DOI 10.55776/FG20
- EU Horizon Europe: Partner in the European Partnership for the Assessment of Risks from Chemicals (PARC) (https://www.eu-parc.eu) Grant DOI 10.3030/101057014)
- EU Horizon 2020: Partner in ALTERNATIVE: Building the Innovative Platform for Detecting Cardiotoxicity of Chemicals. (https://alternative-project.eu/) Grant DOI 10.3030/101037090
- EU Horizon Europe: Partner in CHIASMA: Accessible Innovative Methods for the Safety & Sustainability Assessment of Chemicals and Materials (https://chiasma-project.eu/) Grant-DOI 10.3030/101137613
- EU Horizon Europe: Partner in INSIGHT – Integrated Models for the Development and Assessment of High Impact Chemicals and Materials (https://insight-project.org/) Grant-DOI 10.3030/101137742
- European Food Safety Authority, EFSA: Lead in DNT-RAP4: Pioneering Non Animal Methods-Based Developmental Neurotoxicity Risk Assessment of Pesticides 4] Grant-DOI
Collaborations
- Joyce M Slingerland, Georgetown University, USA
- Richard W. Kriwacki, St. Jude Hosp. of Sick Children, Memphis, USA
- Pierre Roger, Universite Libre de Bruxelles, Brussels, Belgium
- Drorit Neumann, Tel Aviv University, Israel
- Terrance Lappin, Queens University Belfast, UK