Schöpfstraße 41
6020 Innsbruck
Fax: +43 (0)512 9003 73700
Email: Cornelia.Lass-Floerl@i-med.ac.at
Website: https://www.i-med.ac.at/hygiene/
Research year
Research Branch (ÖSTAT Classification)
303002, 303013, 303015, 303020,301902,106024
Keywords
antifungal resistance, complement, dendritic cells, fungal pathogens, hygiene, immunity, infectious diseases, N-chlorotaurine, nosocomial infections, and one health
Research Focus
Understanding infections: from pathogenesis to diagnosis
- Tasks comprise research, teaching, laboratory diagnosis of infectious diseases, hospital services and technical hygiene.
- Scientific activities cover fungal pathogenicity, antifungal resistance, virulence factors, host pathogen-interactions, basic immunological research, fungal carcinogenesis, antimicrobial agents, prevention of nosocomial infections.
- HMM seeks to prevent illness and death from targeted infectious disease threats.
General Facts
Infectious diseases are turning into one of the most frequent causes of death in the world. Understanding the biological principles underlying the mechanisms by which infectious agents adapt and undermine the host defence mechanisms is critical for fighting diseases. HMM is conducting basic and translational research into the molecular mechanisms of the pathogenesis of bacterial, viral and fungal infections and investigating strategies for their prophylaxis and therapy. HMM is bridging the gap between basic and translational research on microbial pathogenesis. Its mission is to coordinate and strategically align translational infection research with the aim of developing new diagnostic, preventative and therapeutic methods to treat infectious diseases. HMM has formed thematic translational units of scientists, each dedicated to a specific pathogen or infectious disease. HMM is one of the largest microbiology diagnostic laboratories in Austria, with an average sample throughput of 250,000 specimens per year. The Institute is associated with all major hospitals in Tyrol, giving it a key position in the diagnostic laboratory landscape in Austria. The group of Wilfried Posch is working on “Exploiting immune response”, while the groups of Michaela Lackner and Cornelia Lass-Flörl are investigating fungal infections, together with Cornelia Speth and Ulrike Binder. Markus Nagl’s main focus is on “N-chlorotaurine”.
Research
Understanding fungal infections: from risk factors and diagnosis to antifungal treatment
Cornelia Lass-Flörl, Ulrike Binder, Roya Vahedi-Shahandashti
Fungal infections represent a significant and growing public health challenge, especially as they are increasingly affecting immunocompromised individuals, such as those with cancer and organ transplantation. The infections can range from superficial to life-threatening, making timely and accurate diagnosis essential for effective treatment. The increasing prevalence of invasive fungal infections (IFIs) and the rise of antifungal resistance highlight the importance of a multifaceted approach to understanding and managing fungal diseases.
Advances in antifungal therapies, including newer drugs such as ibrexafungerp, fosmanogepix and rezafungin, offer potential improvements in efficacy, pharmacokinetics and safety profiles over older agents. It is hoped that the new drugs will address the growing challenge of antifungal resistance, providing more options for treatment, especially in patients with resistant infections. Our ongoing development of antifungal agents continues to focus on broadening spectrum activity, reducing toxicity and improving patient outcomes.
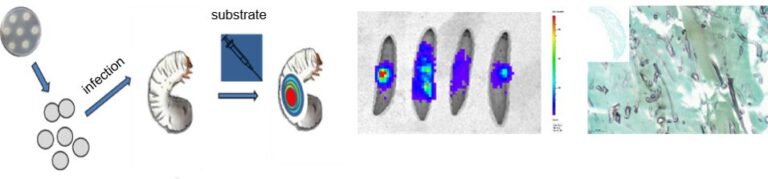
Our group has focused on the epidemiology, diagnosis, prevention and therapy of fungal infections as well as on optimizing the testing for antifungal susceptibility. Understanding the pathogen and why it can cause infection is key to combating disease. We need new in vivo models to study topics such as the role of particular genes in virulence or to test the efficacy of novel antifungal drugs,. In recent years, we have optimized and extensively used the infection model Galleria mellonella in studies of fungal pathogenicity, establishment of disease and the efficacy of new antifungal drugs. We placed a particular emphasis on Mucorales, as the factors and molecular mechanisms that confer virulence, immune evasion and antifungal efficacy are still poorly understood in this group of fungi. With the generation of bioluminescent reporter strains and the simultaneous deletion of genes that may be necessary for virulence, we have another tool available to investigate the onset of infection and the efficacy of antifungal drugs. As pathogenic fungi are increasingly developing resistance to current treatments, and as the current drugs exhibit very high toxicity in humans, there is an urgent need for novel, better drugs. We are currently screening modified antifungal agents in alternative test systems and selecting promising candidates for further analysis. We are also using the luciferase system as an in vivo tool to monitor gene transcription and are focusing on rare moulds as well as on Aspergillus terreus.
Fungal carcinogenesis
Cornelia Speth
Microbe-associated tumorigenesis is a fairly new research area that may open new therapeutic approaches, particularly for tumour types with a poor prognosis. In collaboration with the departments of Surgery and Pathology, the Speth group is focusing on three aspects of fungus-associated carcinogenesis: (1) the detection, identification and quantification of fungi in a selected spectrum of tumour types, combining a range of methods (FISH, PCR, IHC, staining methods); (2) a detailed investigation of the Malassezia-complement interaction to understand how fungi and fungus-induced inflammation might contribute to pancreatic cancer; a knowledge of the molecular processes is the prerequisite for an immune-based therapy that targets the excessive complement activation, and (3) a study of the possibility to eradicate the fungus by optimising antimycotic therapeutic regimens, by investigating the influence of cytostatic drugs on fungal viability and by testing strategies for immunotherapy against the fungus (e.g. CAR-T cells). We are testing these eradication possibilities for Malassezia as well as for other fungal species that might contribute to carcinogenesis.
Targeting Antifungal Resistance from a One Health Perspective
Michaela Lackner
Our research integrates a One Health perspective, emphasizing the interconnected health of humans, animals and the environment. Antifungal agents are widely used in agriculture and medicine, creating overlapping pressures that drive resistance. Understanding the dynamics is essential to mitigating the impact of resistance on clinical outcomes, animal health and ecosystems. In the innovative PhD training course MYCOS, established in 2024 by Lackner and funded by the MUI, we are focusing on antifungal resistance
, addressing the consequences of dual antifungal use and ensuring the sustainability of effective treatments. This holistic approach is critical for combating resistance, protecting public health and preserving environmental balance. As part of the effort, Lackner and her team are deciphering the molecular basis of antifungal resistance to advance drug discovery and development, with a particular emphasis on azole drugs. Azoles target the cytochrome P450 enzyme sterol 14α-demethylase (SDM), which is essential in fungal ergosterol biosynthesis. Resistance mechanisms include efflux pump upregulation, SDM overexpression and ligand-binding pocket modifications. They compromise drug efficacy by altering binding interactions.
We are using Saccharomyces cerevisiae as a heterologous expression system to study the mechanisms, using techniques such as fluorescence microscopy, enzyme assays and crystal structure analysis. We are hoping to develop broad-spectrum azoles with stronger binding affinities, enhanced specificity and reduced resistance and toxicity.
Exploiting the Immune Response
Infection and immunity of emerging pathogens in disease-on-chip models
Wilfried Posch
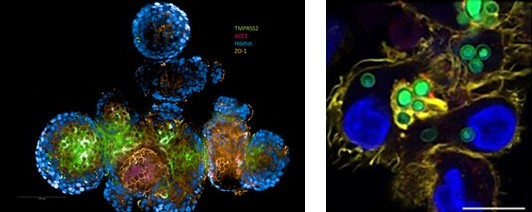
The research group led by Wilfried Posch is focusing on antigen-presenting cells (e.g. dendritic cells (DCs), macrophages) and innate humoral components (e.g. complement) and their interactions with emerging pathogens at barrier sites. The team is investigating the entry mechanisms of various pathogens, including HIV, respiratory viruses, Aspergillus and Candida, and using co-infection models to understand how tissue environments and opsonization patterns influence inflammatory and immune responses. Opsonization severely affects DC functions, such as maturation, anti-viral immunity, T-cell stimulation and activation of restriction factors. The group recently received funding from the FWF to investigate the involvement of cellular complement in the processes, extending the exploration of the role of complement in immune responses upon microbial infection.
Posch oversees a team of MDs from the Institute’s routine diagnostic unit, dedicated to advancing translational and diagnostic research in emerging infectious diseases. The efforts focus on translating basic research into clinical applications, utilizing molecular diagnostics and therapeutic innovations to enhance pathogen detection, treatment and prevention, thereby bolstering global health security and pandemic preparedness. Since the onset of the COVID-19 pandemic, the group has expanded its research to study emerging pathogens such as SARS-CoV-2, focusing on immunity in patients with varying disease severities and responses to vaccines or treatment. The group has analysed both humoral and cellular immunity across patient cohorts, emphasizing neutralization abilities and T-cell specificities against different variants. The studies also compared mucosal immunity and antibody titres in vaccinated and convalescent individuals, revealing differences based on immunization regimens. In addition, we are investigating immune cell crosstalk within mucosal tissues within the CONNECT PhD research training network, funded in December 2022 by the MUI and coordinated by Wilfried Posch. The Posch group has developed and standardized complex human cell culture models, including 3D barrier models and interconnected microphysiological systems, to mimic in vivo conditions and enable the detailed analysis of infection, inflammation, tissue damage and therapeutic targets. Utilizing these immunocompetent 3D models, the group has also explored personalized approaches to studying infection and immunity, allowing rapid responses to emerging infections.
N-chlorotaurine
Markus Nagl
We have synthesized the sodium salt of N-chlorotaurine (NCT), a long-lived oxidant produced by activated human leukocytes, and are investigating it as a new antiseptic for the topical treatment of infections of multiple body regions, including sensitive ones. We are undertaking basic research on its microbicidal activity against emerging pathogens such as mucormycetes and viruses, and its bactericidal activity against planctonic forms and biofilms in surgery and dentistry. In vivo investigations cover the bronchopulmonary system (mechanisms of efficacy of inhaled NCT in mouse fungal pneumonia supported by the FWF; future confirmatory phase II inhalation of NCT in chronic pulmonary diseases such as cystic fibrosis and COPD and acute viral infections planned) and various other topical applications, such as the skin and mucous membranes, the ear-nose-throat region, the urinary tract, the eye and organ abscesses. The advantages of NCT include its high tolerability, a broad spectrum of activity against all strains of pathogens without inducing resistance, the inactivation of virulence factors of pathogens, the enhancement of microbicidal activity in the presence of body fluids and its anti-inflammatory effects.
Laboratory Diagnostics, Hospital and Technical Hygiene
The HMM division fulfils its tasks in the detection and identification of pathogens that cause infections. The work covers bacteriology, parasitology, mycobacteriology and mycology. The diagnostic laboratories are certified according to ISO 9001:2009. Some areas are checked by external audits in accordance with §67 of the Austrian Medicines Law and the FDA, Manufacturing and Product Quality division. The sector of hospital and technical hygiene (accredited under ISO/IEC 17025 and ISO/IEC 17020) is developing guidelines for the prevention of infectious diseases and controlling them in line with the statutory requirements for technical facilities (e.g. disinfection machines).
Pictures
Selected Publications
Lass-Flörl C, Kanj SS, Govender NP, Thompson GR 3rd, Ostrosky-Zeichner L, Govrins MA (2024)
Invasive candidiasis. Nat Rev Dis Primers 10(1):20.
Bellotti R, Speth C, Adolph TE, Lass-Flörl C, Effenberger M, Öfner D, Maglione M (2021)
Micro- and Mycobiota Dysbiosis in Pancreatic Ductal Adenocarcinoma Development
Cancers (Basel). 2021 Jul 8;13(14):3431. doi: 10.3390/cancers13143431. PMID: 34298645
Toepfer S, Lackner M, Keniya MV, Monk BC. Functional Expression of Recombinant Candida auris Proteins in Saccharomyces cerevisiae Enables Azole Susceptibility Evaluation and Drug Discovery. J Fungi (Basel). 2023 Jan 27;9(2):168. doi: 10.3390/jof9020168. PMID: 36836283
Diem G, Lafon E, Bauer A, Lass-Flörl C, Reindl M, Wilflingseder D, Posch W. Salivary IgAs and Their Role in Mucosal Neutralization of SARS-CoV-2 Variants of Concern. J Clin Microbiol. 2022 Sep 21;60(9):e0106522. doi: 10.1128/jcm.01065-22. Epub 2022 Aug 29. PMID: 36036600
Kowalczyk K, Coraça-Huber DC, Wille-Kolmar W, Berktold M and Nagl M. Activity of N-chlorotaurine against periodontal pathogens. Int J Mol Sci. 2024 Jul 30; 25(15): 8357. doi: 10.3390/ijms25158357. PMID: 39125925
Štepánek O, Parigger M, Procházková E, Čmoková A, Kolařík M, Dračínská H, Černá V, Kalíková K, Grobárová V, Černý J, Scheler J, Schweiger G, Binder U*, Ondřej Baszczyňski O. Prodrugging fungicidal Amphotericin B significantly decreases its toxic effects. Eur J Med Chem. 2025 Feb 5;283:117157. Epub 2024 Feb 12. PMID: 38345272
Lax C, Mondo S, Osorio-Concepción M, Muszewska A, Corrochano-Luque M, Gutiérrez G, Riley R, Lipzen A, Guo J, Hundley H, Amirebrahimi M, Ng V, Lorenzo-Gutierrez D, Binder U, Yang J, Song Y, Cánovas D, Navarro E, Freitag M, Gabaldon T, Grigoriev I, Corrochano L, Nicolás F and Garre V. Nat Commun. 2024 Jul 18;15(1):6066
Klimt M, Stadler M, Binder U*, Krauss J. Synthesis of novel benzylamine antimycotivs and evaluation of their antimycotic potency. Arch Pharm (Weinheim). 2024 May;357(5):e2300381 (shared corresponding)
Osorio-Concepción M, Lax C, Lorenzo-Gutiérrez D, Tahiri G, Cánovas-Márquez JT, Navarro E, Binder U, Nicolás FE, Garre V. H3K4 methylation regulates development, DNA repair, and virulence in Mucorales. IMA Fungus. 2024 Mar 14;15(1):6.
Scheler J, Binder U*. Alternative in-vivo models of mucormycosis. Front Cell Infect Microbiol. 2024 Feb 1; 14:1343834
Eisele D, Blatzer M, Dietl AM, Binder U, Müller C, Hagen F, Sae-Ong T, Schäuble S, Panagiotou G, Vahedi-Shahandashti R, Lass-Flörl C (2025) Aspergillus terreus sectorization: a morphological phenomenon shedding light on amphotericin B resistance mechanism. mBio 16:e03926-24.
Lafon E, Jäger M, Bauer A, Reindl M, Bellmann-Weiler R, Wilflingseder D, Lass-Flörl C, Posch W. Comparative analyses of IgG/IgA neutralizing effects induced by threee COVID-19 vaccines against variants of concern. J Allergy Clin Immunol. 2022 Apr;149(4):1242-1252. PMID: 35093484
Dichtl S, Posch W, Wilflingseder D. The breathtaking world of human respiratory in vitro models: Investigating lung diseases and infections in 3D models, organoids, and lung-on-chip. Eur J Immunol. 2024 Mar;54(3):e2250356. doi: 10.1002/eji.202250356. Epub 2024 Feb 15. PMID: 38361030
Zaderer V, Diem G, Posch W, Jakschitz T, Bonn GK, Bellmann-Weiler R, Huber LA, Wilflingseder D. P80 natural essence spray and lozenges provide respiratory protection against Influenza A, B, and SARS-CoV-2. Respir Res. 2024 Feb 28;25(1):102. doi: 10.1186/s12931-024-02718-0. PMID: 38419061
Grubwieser P, Böck N, Soto EK, Hilbe R, Moser P, Seifert M, Dichtl S, Govrins MA, Posch W, Sonnweber T, Nairz M, Theurl I, Trajanoski Z, Weiss G. Human airway epithelium controls Pseudomonas aeruginosa infection via inducible nitric oxide synthase Front Immunol. 2024 Dec 3;15:1508727. doi: 10.3389/fimmu.2024.1508727. eCollection 2024. PMID: 39691712
Schatz C, Knabl L, Lee HK, Seeboeck R, von Laer D, Lafon E, Borena W, Mangge H, Prüller F, Qerimi A, Wilflingseder D, Posch W, Haybaeck J. Machine Learning to Identify Critical Biomarker Profiles in New SARS-CoV-2 Variants Microorganisms. 2024 Apr 15;12(4):798. doi: 10.3390/microorganisms12040798. PMID: 38674742
Sturm A, Jóźwiak G, Verge MP, Munch L, Cathomen G, Vocat A, Luraschi-Eggemann A, Orlando C, Fromm K, Delarze E, Światkowski M, Wielgoszewski G, Totu RM, García-Castillo M, Delfino A, Tagini F, Kasas S, Lass-Flörl C, Gstir R, Cantón R, Greub G, Cichocka D. Accurate and rapid antibiotic susceptibility testing using a machine learning-assisted nanomotion technology platform. Nat Commun. 2024, 15(1):2037.
Govrins M, Lass-Flörl C. Candida parapsilosis in the clinical setting. Nat Rev Microbiol. 2024; 22(1):46-59.
van Rhijn N, Arikan-Akdagli S, Beardsley J, Bongomin F, Chakrabarti A, Chen SC, Chiller T, Lopes Colombo A, Govender NP, Alastruey-Izquierdo A, Kidd SE, Lackner M, Li R, Hagen F. Beyond bacteria: the growing threat of antifungal resistance. Lancet. 2024 Sep 14;404(10457):1017-1018. doi: 10.1016/S0140-6736(24)01695-7.
Niu X, Al-Hatmi AMS, Vitale RG, Lackner M, Ahmed SA, Verweij PE, Kang Y, de Hoog S. Evolutionary trends in antifungal resistance: a meta-analysis. Microbiol Spectr. 2024 Apr 2;12(4):e0212723. doi: 10.1128/spectrum.02127-23. Epub 2024 Mar 6.
Toepfer S, Keniya MV, Lackner M, Monk BC. Azole Combinations and Multi-Targeting Drugs That Synergistically Inhibit Candidozyma auris. J Fungi (Basel). 2024 Oct 7;10(10):698. doi: 10.3390/jof10100698.
Schoberleitner I, Baier L, Lackner M, Zenz LM, Coraça-Huber DC, Ullmer W, Damerum A, Faserl K, Sigl S, Steinkellner T, Winkelmann S, Sarg B, Egle D, Brunner C, Wolfram D. Surface Topography, Microbial Adhesion, and Immune Responses in Silicone Mammary Implant-Associated Capsular Fibrosis. Int J Mol Sci. 2024 Mar 9;25(6):3163. doi: 10.3390/ijms25063163.
Fiala J, Roach T, Holzinger A, Husiev Y, Delueg L, Hammerle F, Armengol ES, Schöbel H, Bonnet S, Laffleur F, Kranner I, Lackner M, Siewert B. The Light-activated Effect of Natural Anthraquinone Parietin against Candida auris and Other Fungal Priority Pathogens. Planta Med. 2024 Jun;90(7-08):588-594. doi: 10.1055/a-2249-9110. Epub 2024 Jun 6.
Selection of Funding
- CD-Labor für Invasive Pilzinfektionen; 2015-2022
- FWF, Doctoral programme of excellence, “HOROS”, ZFW012530, Host response in opportunistic infections, 2014-2023
- EU, Horizon2020, Marie Sklodowska Curie Action European Joint Doctorate “CORVOS”, 860044, Complement regulation & variations in opportunistic infections. 2019-2024
- FWF-Einzelprojekt P32329 Titel: Primäre Azoleresistenz in Mucormyzeten; 2019-2024
- MUI-Prototypenförderung 2022 Titel: SolNail; 2023
- FWF P33510: HIV-C entflieht Restriktion, nicht Sensing in DCs; 2020-2024
- FWF P34070: Treating SARS-CoV-2 infection in human 3D respiratory models; 2020-2024
- FWF P32329-B: Intrinsic azole resistance in mucormycetes; 2019-2023
- Fungal Dysbiosis in Pancreatic Ductal Adenocarcinoma Progression – The crosstalk with tumor microenvironment and its clinical implications. In Memoriam Dr. Gabriel Salzner Privatstiftung; 2021-2023
- FWF-Einzelprojekt P36922: Malassezia-induced immune-environment of pancreatic ductal adenocarcinoma; 2023-2026
- FWF-Einzelprojekt PAT7129523; 2025-2028: NCT for therapy and prophylaxis of airborne mycoses
Collaborations
Peter Garred, HOROS Guest Professor, Rikshospitalet, University of Copenhagen, Denmark
Maurizio Brigotti, University of Bologna, Italy
Brian Monk, Department of Oral Sciences, Division of Health Sciences, University of Otago, Dunedin, New Zealand
Erwin Lamping, Sir John Walsh Research Institute, Faculty of Dentistry, University of Otago, Dunedin, New Zealand
Oliver T. Keppler, Ludwig-Maximilians-Universität München, Munich, Germany
Teunis BH Geijtenbeek, Amsterdam UMC, Amsterdam Institute for Infection & Immunity, Amsterdam, The Netherlands
Oliver Cornely, Klinik I für Innere Medizin, Uniklinik Köln, Köln, Germany
Carsten Schwarz, CF Center Westbrandenburg, Campus Potsdam, Potsdam, Germany