Peter-Mayr-Str. 1
6020 Innsbruck
Fax: +43 512 9003 70502
Email: humgen@i-med.ac.at
Website: http://www.humgen.at/
Research Branch (ÖSTAT Classification)
Keywords
Biochemical Genetics, Dental genetics, Ehlers-Danlos syndromes, Human Genetics, inherited metabolic disorders, Inherited tumour disposition syndromes, Lipidomics, Neurogenetics, Reproductive medicine, Syndromology, and Tumour genetics
Research Focus
The primary aim of the Institute of Human Genetics is the clarification of the genetic determinants of health and disease in humans, and to translate this knowledge into individual medical care. This aim is achieved by combining comprehensive clinical services and all relevant laboratory diagnostic techniques with basic research. The main focus is on rare diseases that are inherited as monogenic traits with expansion to polygenic disease risk factors. The institute includes the Centre for Medical Genetics Innsbruck, which provides medical genetic services and genetic counselling for Western Austria with extensive outpatient clinics and inpatient consultation in Innsbruck and several regional hospitals. The diagnostic and research laboratories cover all relevant methods for molecular genetic (DNA, RNA, epigenetics) and cytogenetic (genomic) analyses, biochemical analyses with a focus on lipidomics and a wide range of functional and cell biology techniques. The institute is dedicated to interdisciplinary collaboration and shares research activities with a large number of cooperation partners.
General Facts
Research
Johannes Zschocke
Elucidating the link between the genetic information and phenotype characteristics is the primary focus of Human Genetics; Medical Genetics applies this knowledge to the diagnosis and management of human diseases. On the 200th anniversary of Gregor Mendel’s birthday in 2022, we published an essay on the concepts of dominant and recessive for understanding inheritance, and the differences in the definition of these terms by Mendel and in genetic medicine. A subsequent major review article examined the different quantitative and qualitative effects of genetic variants and their relevance to understanding and treating inherited monogenic diseases.
Biochemical Genetics
Markus Keller, Johannes Zschocke
We focus on characterising clinical, biochemical and molecular features of inherited metabolic disorders, including various aspects of metabolism such as lipids, amino acids, and organic acids. We developed the International Classification of Inherited Metabolic Disorders (ICIMD), a comprehensive collection of over 1400 monogenic diseases. This classification is endorsed by international societies for inherited metabolic diseases and serves as the basis for the widely used handbook, the 5th Edition of the Vademecum Metabolicum.
Biochemical Genetics Laboratory
In our Biochemical Genetics Laboratory we integrate genetic and multi-omics data using bioinformatics to understand genetically determined diseases. Our focus is on lipid metabolic processes and their impact on various diseases. We specialise in lipidomics, the study of how metabolic diseases affect membrane lipids and enzymatic processes. Our goal is to develop new therapies and analysis methods for inherited metabolic disorders.
Regulation of Lipid Metabolism in Mitochondrial Membranes:
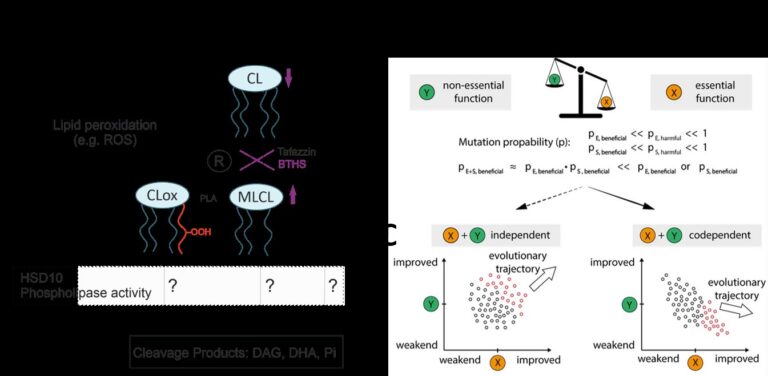
Mitochondrial membrane lipid metabolism is vital for optimal mitochondrial function. Dysregulation can cause inherited metabolic disorders like Barth syndrome (BTHS), characterised by severe cardiomyopathy. Changes in cardiolipin composition, a specific phospholipid, are observed in BTHS (e.g. Oemer et al. 2022) and different other conditions (e.g. Gonzalez-Franquesa et al. 2022). Understanding the role of lipid metabolism in mitochondrial function reveals potential therapeutic targets, while considering the influence of fatty acid metabolism on lipid composition (Keller et al. 2021).
Developing novel LC-MS/MS based lipidomics approaches:
Accurate characterisation of complex lipid compositions is crucial for studying lipid metabolism. We develop specialised LC-MS/MS techniques with optimised parameters and fragmentation strategies to identify and quantify challenging lipids like cardiolipins, plasmalogens and ether lipids. These advancements enhance our understanding of their functional roles in cellular processes (e.g. as summarised in Koch et al. 2022).
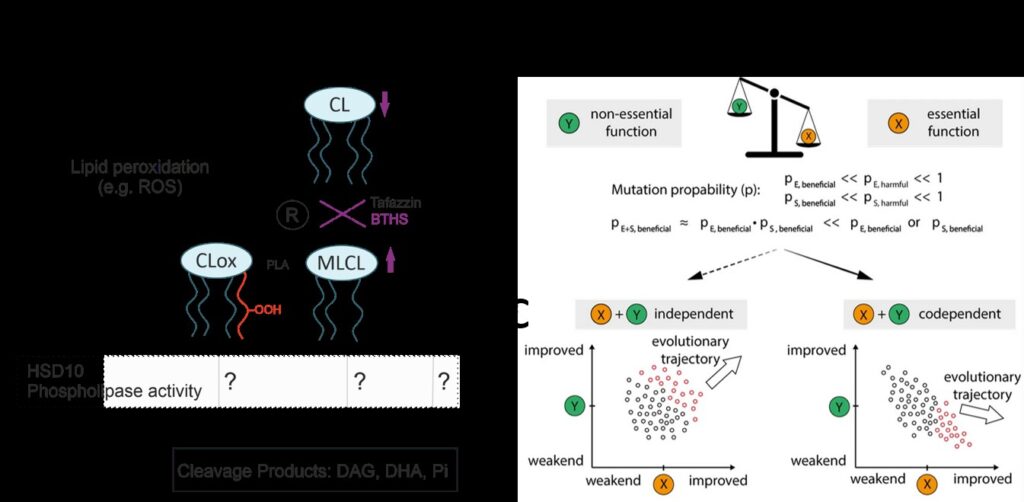
Enzymology and evolutionary principles of HSD10:
HSD10 is an essential mitochondrial protein with different functions and a significant evolutionary history. Mutations in the HSD17B10 gene cause HSD10 disease, associated with cardiomyopathy and neurodegeneration. We show that previous reports on HSD10’s cardiolipin-cleaving activity are most likely an in vitro artifact (Wohlfarter et. al. 2022). As a crucial part of the mitochondrial RNase P complex, HSD10s evolutionary trajectory is constrained, thereby potentially impacting substrate specificity and reaction rates. Understanding these evolutionary principles is important for comprehending HSD10 substrate promiscuity and function.
Monogenic and Polygenic Hypercholesterolaemia
Gunda Schwaninger, Martina Witsch-Baumgartner, Johannes Zschocke
We are interested in the genetic basis of Familial Hypercholesterolaemia (FH) in Austria and in the functional impact of genetic factors on disease severity and phenotype. In addition to the known monogenic causes, we are investigating polygenic causes of hypercholesterolaemia in a cohort of >250 well-characterised individuals from the Austrian FH register, in cooperation with the University Hospital for Internal Medicine I at MUI. The identification of genetic scores has been established using the DNA array technology (Illumina iScan) in the institute in close collaboration with the Institute of Genetic Epidemiology at MUI. Integrating genetic scores for LDL-C and Lp(a) increases the proportion of hypercholesterolemia patients with a clearly defined disease aetiology to 68.8% compared to 46.6% in standard genetic testing. These studies return promising insights into optimising individualised testing, therapeutic approaches, lifestyle counselling and risk assessment with regard to cardiovascular events.
Hereditary Cancer Genetics
Katharina Wimmer, Simon Schnaiter, Esther Schamschula
The mission of the Hereditary Cancer Genetics group is the improvement of cancer predisposition syndrome (CPS) diagnostics, to identify novel genetic causes of CPS and to uncover the molecular mechanisms of tumorigenesis in patients with CPS. The two main topics of the past years were:
Genetic and clinical characterisation of constitutional mismatch repair deficiency (CMMRD) and related disorders
CMMRD caused by bi-allelic mutations in one of the four MMR genes is associated with a high propensity for a broad spectrum of malignancies in childhood and adolescence. Under our lead, the European consortium Care for CMMRD (C4CMMRD) developed criteria for the clinical suspect diagnosis in paediatric cancer patients. Another CMMRD diagnostics’ milestone achieved in collaboration with the Newcastle University was the development of a highly reliable assay testing for constitutional microsatellite instability (cMSI) in blood lymphocyte’s DNA, which is a hallmark feature of CMMRD. With this assay, we can nowadays come to a definite diagnosis that either confirms or refutes CMMRD in a suspected patient and allows us to uncover novel genetic aetiologies in suspected CMMRD patients in whom this diagnosis is excluded.
By extensive germline and tumour analyses, we were able to prove that specific de novo constitutional heterozygous POLE mutations and di-genic inheritance of a heterozygous PMS2 and a heterozygous POLD1 germline mutation are two alternative causes of a CMMRD-like phenotype. The severe clinical phenotypes of our cases render new insights the penetrance of POLE/POLD1 variants that may be modulated by a high risk for somatic MMR deficiency. Together with additional simultaneously published cases, our cases represent new forms of constitutional replication repair deficiencies.
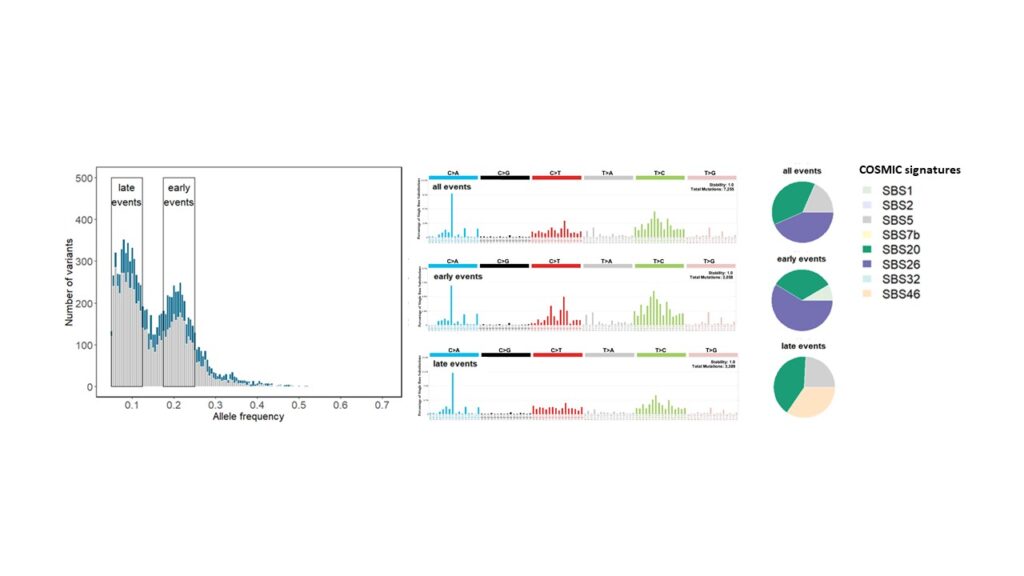
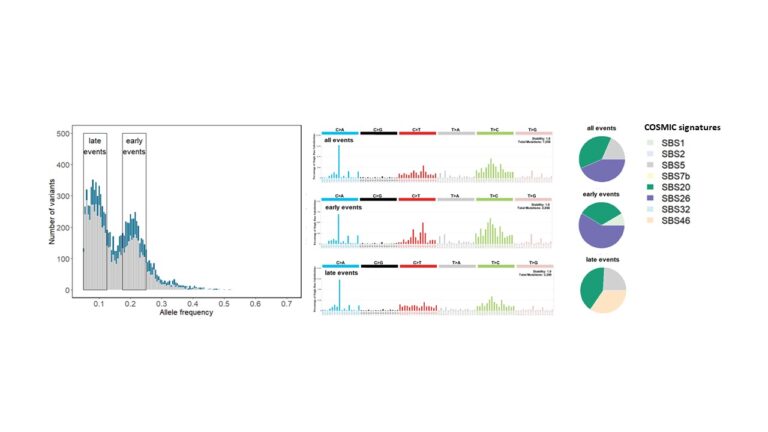
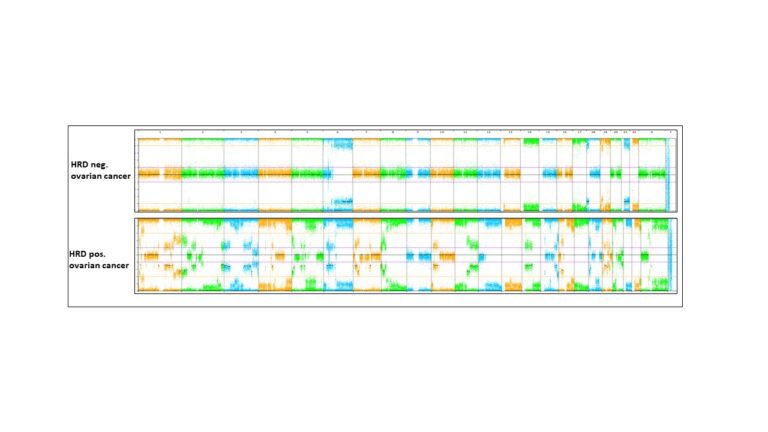
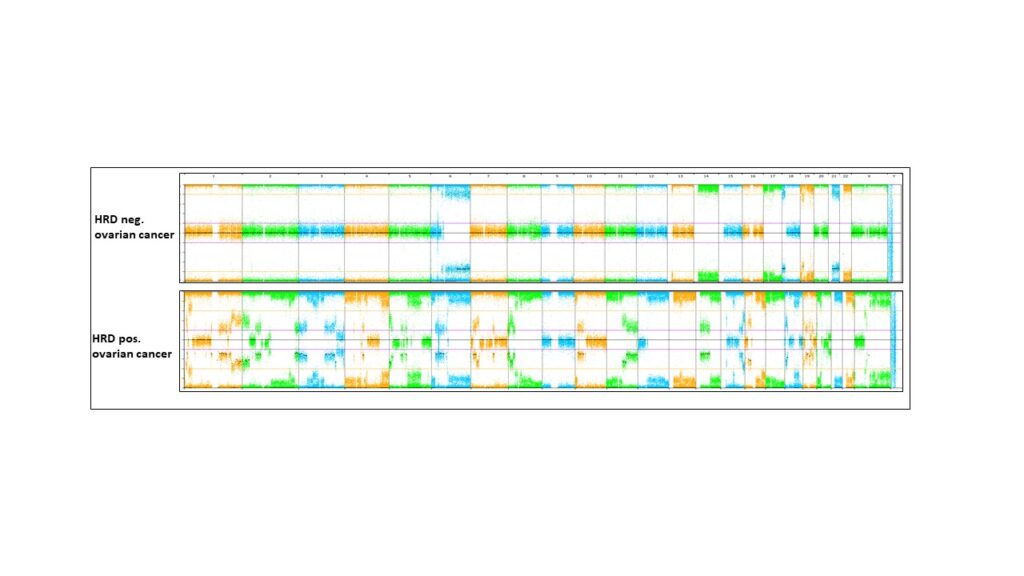
Genomic instability in ovarian cancer and other tumours
Genomic instability caused by homologous recombination deficiency (HRD) is a predictive biomarker for the response of different cancer types to PARP-inhibitor and platinum-based therapies. HRD results from the loss of function of genes involved in homologous recombination repair (HRR), but probably also from yet unknown mechanisms. Assessment of HRD, independent of the underlying variable causing the mechanism, can be performed by detection of characteristic genomic instability resulting from the impaired HRR.
As a part of our tumour profiling platform, we implemented and validated a simple and cost-effective method for the assessment of genomic instability. Next, amongst other methods, we will use machine learning to delineate the impact of genomic instability and copy number alterations on tumorigenesis, tumour phenotype and therapy response including and beyond HRD in ovarian cancer and other tumour entities.
Genetic and Epigenetic Alterations in Haematological Malignancies and Solid Tumours
Emina Jukic, Simon Schwendinger, Verena Vogi
Since 2019, we have been working on genetic and epigenetic alterations in haematological malignancies and specific solid tumours.
Collaboration with Innsbruck University Hospital for Internal Medicine V
Together with the Austrian Myeloma Registry, we investigated genetic variants contributing to the heterogeneity of relapsed multiple myeloma (MM). Cytogenetic progression is common and amplification of 1q is found in the disease progression.
Furthermore, we focus on the investigation of epigenetic alterations in MDS together with the Institute of Medical Genetics and Pathology in Basel and the Institute of Human Genetics in Bonn. DNA methylation is an important, in cancer cells often dysregulated, epigenetic mechanism for gene regulation. We investigate the methylome in a clinical setting for diagnosis, stratification and therapy.
Moreover, we investigated Clonal haematopoiesis of indeterminate potential (CHIP) in COVID-19 patients together with the Univ.-Hospital Internal Medicine II. CHIP reflects the expansion of a blood cell clone due to acquired somatic mutations without leading to malignancy in an inflammatory environment. CHIP in COVID-19 patients was stable and not linked to an aggravated clinical course.
Collaboration with Innsbruck University Hospital for Internal Medicine IV
We also study CHIP in other inflammation-associated diseases, in this case concerning patients with type 2 diabetes mellitus. Common risk factors and elevated micro-inflammation, but not CHIP, was associated with the decline of kidney function.
Collaboration with Innsbruck University Hospital for Internal Medicine II
Polygenic Scores (PGS) are suspected to predict disease as well as disease courses. Together with the Institute of Genetic Epidemiology, we focus on a PGS for CRP and disease severity in COVID-19 outcomes.
Collaboration with Innsbruck University Hospital for Dermatology, Venereology and Allergology
Furthermore, we investigate CHIP in the context of Atopic Dermatitis (AD). The main goal of the project is to determine the role and prevalence of lymphatic CHIP in AD, as patients with AD are more likely to develop lymphomas.
In addition, we are assessing the genetic and epigenetic – including methylome – status in malignant melanoma for a more precise disease characterisation and to distinguish malignant lesions from benign melanocytic nevi.
Collaboration with Visceral-Oncologic Center at Ordensklinikum Linz
In collaboration with the Visceral-Oncologic Center in Linz, we investigated the role of circulating tumour DNA (ctDNA) detected before therapy as a prognostic biomarker in patients with metastatic pancreatic cancer. An analysis using liquid biopsy prospectively and serially revealed that change in magnitude of ctDNA (below 60% of baseline) during treatment adds significant predictive clinical potential. ctDNA also predicts the outcome in localised as well as disseminated disease in pancreatic ductal cancer (PDAC), undetectable by current gold standard methods.
Periodontal Ehlers-Danlos syndrome and other inherited connective tissue disorders
Johannes Zschocke, Albert Amberger
Ehlers-Danlos syndromes (EDS) are a group of inherited connective tissue disorders characterised by joint hypermobility, skin hyper-elasticity and the fragility of various tissues. We have a long track record in the identification and functional characterisation of monogenic EDS types. The most recent focus was on elucidation of the pathogenetic mechanism that causes periodontal EDS (pEDS). The defining clinical feature of pEDS is rapidly progressive periodontitis and the loss of teeth at a young age; the condition is caused by heterozygous missense or in-frame insertion/deletion mutations in the genes for complement 1 subunits C1r and C1s. Extensive functional studies of pEDS-associated C1r variants showed that the central elements in the pathogenesis of pEDS seem to be intracellular activation of C1r and C1s and extracellular presence of activated C1s independently of microbial triggers.
Recently, we identified activated C1s (aC1s) as an enzyme that degrades collagen I in patient cells and in in vitro assays. Interestingly, collagen I was completely degraded when assays were performed at 40°C, indicating that moderately elevated temperature has an impact on the integrity of collagen I. Degraded collagen I is expected to interfere with the compaction process that is important for mature tissue function resulting in loosened tissue, a hallmark of the EDS phenotype. Our results indicate that pathogenesis in pEDS is mediated by inadequate activation of C1s and subsequent C1s-mediated degradation of matrix proteins, confirming pEDS as a connective tissue disorder.
Neurogenetics
Sabine Rudnik
The institute is a member of the German CMT Network supported by the German Ministry of Education and Research from 2016-2019. Inherited peripheral neuropathies are the most common hereditary neuromuscular diseases. Within the CMT-Network, we updated the genetic diagnostic algorithms, studied the outcome of pregnancy and delivery in patients with CMT disease and are currently updating the AWMF guidelines for peripheral neuropathies in childhood.
Reproductive Medicine
Sabine Rudnik, Johannes Zschocke
In 2018 and 2019 the first guidelines in German-speaking countries were published on diagnostics and therapeutic procedures of recurrent miscarriage and on diagnosis and treatment before assisted reproduction. In 2021 the guidelines for couples with recurrent miscarriage were updated und published in August 2022 (AWMF S2K-Leitlinie 015/050). The most important recommendations were summarised for clinical geneticists (Wyrwoll et al. 2021). Moreover, we started an initiative to implement preconception carrier screening (PCS) in Austria. We hosted an international workshop entitled “Implementation of Preconception Carrier Screening in Austria” in November 2022 and published the current status of PCS in Europe (Rudnik-Schöneborn and Zerres 2022).
Genetic and Genomic Counselling
Gunda Schwaninger, Johannes Zschocke, Sabine Rudnik
The rapid development of genetic diagnostic methods leads to an increased need for competent professionals who can convey the background, goals, methods, results and consequences of genetic testing in the context of individual decision-making. Master-trained Genetic Counsellors have become widely integrated internationally, but are only recently established in the German-speaking countries. Genetic Counsellors form a part of the inter-professional medical genetics team and complement patient care, particularly in psychosocial counselling aspects. In 2019, we established the first German-taught MSc programme in Genetic and Genomic Counselling at the MUI. The third 5-semester course has started in September 2023. In parallel, we are conducting research projects on the perception and optimal integration of this new profession within Austrian, German and Swiss genetic services. Further information is available at https://www.i-med.ac.at/gencouns/.
European Reference Networks for Rare Diseases
Johannes Zschocke, Sabine Rudnik, Katharina Wimmer, Christine Fauth
In 2019, the institute was appointed Associated Centre of four European Reference Networks (ERNs): ReCONNET for connective tissue and musculoskeletal diseases, EURO-NMD for neuromuscular diseases, GENTURIS for genetic tumour risk syndromes, and ITHACA for congenital malformations and rare intellectual disability. In addition, the institute participates in several other Associated Centres established at the MUI and co-directs the Centre for Rare Diseases Innsbruck. Many rare diseases have a primary genetic origin, and medical genetics plays a major role in diagnosis, management and research of these conditions. This requires extensive international networking. For example, within the EURO-NMD network we published a series of patients with a rare subtype of nemaline myopathy caused by Austrian and Bavarian founder LMOD3 mutations. We were also able to define the genetic diagnosis in patients with unexplained myopathy by means of exome sequencing within the MYO-SEQ Consortium.
Pictures
Selected Publications
General topics:
- Zschocke J, Byers PH, Wilkie AOM. Mendelian inheritance revisited: dominance and recessiveness in medical genetics. Nat Rev Genet. 2023 Feb 20. doi: 10.1038/s41576-023-00574-0. Epub ahead of print. PMID: 36806206.
- Zschocke J, Byers PH, Wilkie AOM. Gregor Mendel and the concepts of dominance and recessiveness. Nat Rev Genet. 2022 Jul;23(7):387-388. doi: 10.1038/s41576-022-00495-4. PMID: 35508637.
Biochemical Genetics:
- Keller, M. A. (2021). Interpreting phospholipid and cardiolipin profiles in rare mitochondrial diseases. Current Opinion in Systems Biology, 28(Icimd), 100383. https://doi.org/10.1016/j.coisb.2021.100383
- Koch, J., Watschinger, K., Werner, E. R., & Keller, M. A. (2022). Tricky Isomers—The Evolution of Analytical Strategies to Characterize Plasmalogens and Plasmanyl Ether Lipids. Frontiers in Cell and Developmental Biology, 10(April), 1–13. https://doi.org/10.3389/fcell.2022.864716
- Oemer, G., Edenhofer, M.-L., Wohlfarter, Y., Lackner, K., Leman, G., Koch, J., Cardoso, L. H. D., Lindner, H. H., Gnaiger, E., Dubrac, S., Zschocke, J., & Keller, M. A. (2021). Fatty acyl availability modulates cardiolipin composition and alters mitochondrial function in HeLa cells. Journal of Lipid Research, 62, 100111. https://doi.org/10.1016/j.jlr.2021.100111
- Oemer, G., Koch, J., Wohlfarter, Y., Lackner, K., Gebert, R. E. M., Geley, S., Zschocke, J., & Keller, M. A. (2021). The lipid environment modulates cardiolipin and phospholipid constitution in wild type and tafazzin-deficient cells. Journal of Inherited Metabolic Disease, n/a(n/a). https://doi.org/10.1002/jimd.12433
- Wohlfarter, Y., Eidelpes, R., Yu, R. D., Sailer, S., Koch, J., Karall, D., Scholl, S., Albert, B., Hauke, A., Zschocke, J., & Keller, M. A. (2022). Lost in promiscuity ? An evolutionary and biochemical evaluation of HSD10 function in cardiolipin metabolism. Cellular and Molecular Life Sciences, 1, 1–13. https://doi.org/10.1007/s00018-022-04579-6
Hereditary Cancer Genetics:
- Constitutional Microsatellite Instability, Genotype, and Phenotype Correlations in Constitutional Mismatch Repair Deficiency. Gallon R, Phelps R, Hayes C, Brugieres L, Guerrini-Rousseau L, Colas C, Muleris M, Ryan NAJ, Evans DG, Grice H, Jessop E, Kunzemann-Martinez A, Marshall L, Schamschula E, Oberhuber K, Azizi AA, Baris Feldman H, Beilken A, Brauer N, Brozou T, Dahan K, Demirsoy U, Florkin B, Foulkes W, Januszkiewicz-Lewandowska D, Jones KJ, Kratz CP, Lobitz S, Meade J, Nathrath M, Pander HJ, Perne C, Ragab I, Ripperger T, Rosenbaum T, Rueda D, Sarosiek T, Sehested A, Spier I, Suerink M, Zimmermann SY, Zschocke J, Borthwick GM, Wimmer K, Burn J, Jackson MS, Santibanez-Koref M. Gastroenterology. 2022 Dec 29:S0016-5085(22)01444-5. doi: 10.1053/j.gastro.2022.12.017. Online ahead of print. PMID: 36586540
- Teenage-Onset Colorectal Cancers in a Digenic Cancer Predisposition Syndrome Provide Clues for the Interaction between Mismatch Repair and Polymerase δ Proofreading Deficiency in Tumorigenesis. Schamschula E, Kinzel M, Wernstedt A, Oberhuber K, Gottschling H, Schnaiter S, Friedrichs N, Merkelbach-Bruse S, Zschocke J, Gallon R, Wimmer K. Biomolecules. 2022 Sep 22;12(10):1350. doi: 10.3390/biom12101350. PMID: 36291559
- Constitutional POLE variants causing a phenotype reminiscent of constitutional mismatch repair deficiency. Sehested A, Meade J, Scheie D, Østrup O, Bertelsen B, Misiakou MA, Sarosiek T, Kessler E, Melchior LC, Munch-Petersen HF, Pai RK, Schmuth M, Gottschling H, Zschocke J, Gallon R, Wimmer K. Hum Mutat. 2022 Jan;43(1):85-96. doi: 10.1002/humu.24299. Epub 2021 Dec 2. PMID: 34816535
- Genotype-phenotype associations in a large PTEN Hamartoma Tumor Syndrome (PHTS) patient cohort. Hendricks LAJ, Hoogerbrugge N, Venselaar H, Aretz S, Spier I, Legius E, Brems H, de Putter R, Claes KBM, Evans DG, Woodward ER, Genuardi M, Brugnoletti F, van Ierland Y, Dijke K, Tham E, Tesi B, Schuurs-Hoeijmakers JHM, Branchaud M, Salvador H, Jahn A, Schnaiter S, Anastasiadou VC, Brunet J, Oliveira C, Roht L, Blatnik A, Irmejs A; PTEN Study Group; Mensenkamp AR, Vos JR. Eur J Med Genet. 2022 Dec;65(12):104632. doi: 10.1016/j.ejmg.2022.104632. Epub 2022 Oct 18. PMID: 36270489
- Low-level mosaicism in tuberous sclerosis complex in four unrelated patients: Comparison of clinical characteristics and diagnostic pathways. Manzanilla-Romero HH, Weis D, Schnaiter S, Rudnik-Schöneborn S. Am J Med Genet A. 2021 Dec;185(12):3851-3858. doi: 10.1002/ajmg.a.62433. Epub 2021 Jul 30. PMID: 34328706
Periodontal Ehlers-Danlos syndrome and other inherited connective tissue disorders:
- Degradation of collagen I by activated C1s in periodontal Ehlers-Danlos Syndrome. Amberger, A., Pertoll, J. Traunfellner, P. Kapferer-Seebacher, I. Stoiber, H. Klimaschewski, L. Thielens, N. Gaboriaud, C. Zschocke, J. Front Immunol. 2023 Mar 7;14:1157421. doi: 10.3389/fimmu.2023.1157421. PMC10028100.
Genetic and Genomic Counselling:
- The genetic counseling profession in Austria: Stakeholders’ perspectives. Schwaninger G, Benjamin C, Rudnik-Schöneborn S, Zschocke J. J Genet Couns. 2021 Jun;30(3):861-871. doi: 10.1002/jgc4.1389. Epub 2021 Apr 2. PMID: 33797821; PMCID: PMC8251832
- Prospects and challenges for the genetic counsellor profession in the German-speaking countries: report of a workshop.Schwaninger, Gunda, Heidemann, Simone, Hofmann, Wera, Maurer, Tamara, Mayerhanser, Katharina, Ronez, Joelle, Schüler, Herdit, Steinmüller, Katharina, Rudnik-Schöneborn, Sabine and Zschocke, Johannes. Medizinische Genetik, vol. 33, no. 1, 2021, pp. 35-44. https://doi.org/10.1515/medgen-2021-2055
- Schwaninger G., Forer L., Ebenbichler C., Dieplinger H., Kronenberg F., Zschocke J., Witsch-Baumgartner M. Filling the gap: Genetic risk assessment in hypercholesterolemia using LDL-C and LPA genetic scores. Clinical Genetics. 2023; 1- 10. doi:1111/cge.14387
Monogenic and Polygenic Hypercholesterolaemia:
- Array genotyping as diagnostic approach in medical genetics.
Witsch-Baumgartner M, Schwaninger G, Schnaiter S, Kollmann F, Burkhard S, Gröbner R, Mühlegger B, Schamschula E, Kirchmeier P, Zschocke J. Mol Genet Genomic Med. 2022 Sep;10(9):e2016. doi: 10.1002/mgg3.2016. Epub 2022 Aug 1. PMID: 35912641; PMCID: PMC9482391.
Genetic and Epigenetic Alterations in Haematological Malignancies and Solid Tumors:
- Locher M, Jukic E, Vogi V, Keller MA, Kröll T, Schwendinger S, Oberhuber K, Verdorfer I, Mühlegger BE, Witsch-Baumgartner M, Nachbaur D, Willenbacher W, Gunsilius E, Wolf D, Zschocke J, Steiner N. Amp(1q) and tetraploidy are commonly acquired chromosomal abnormalities in relapsed multiple myeloma. Eur J Haematol. 2023 Mar;110(3):296-304. doi: 10.1111/ejh.13905. Epub 2022 Dec 4. PMID: 36433728
- Vogi V, Denicolò S, Mayer G, Zschocke J, Jukic E. Response. Kidney Int Rep. 2022 Aug 27;7(11):2543-2544. doi: 10.1016/j.ekir.2022.08.015. PMID: 36531870; PMCID: PMC9751569.
- Kirchweger P, Kupferthaler A, Burghofer J, Webersinke G, Jukic E, Schwendinger S, Wundsam H, Biebl M, Petzer A, Rumpold H. Prediction of response to systemic treatment by kinetics of circulating tumor DNA in metastatic pancreatic cancer. Front Oncol. 2022 Aug 30;12:902177. doi: 10.3389/fonc.2022.902177. PMID: 36110940; PMCID: PMC9468369.
- Kirchweger P, Kupferthaler A, Burghofer J, Webersinke G, Jukic E, Schwendinger S, Weitzendorfer M, Petzer A, Függer R, Rumpold H, Wundsam H. Circulating tumor DNA correlates with tumor burden and predicts outcome in pancreatic cancer irrespective of tumor stage. Eur J Surg Oncol. 2022 May;48(5):1046-1053. doi: 10.1016/j.ejso.2021.11.138. Epub 2021 Dec 1. PMID: 34876329.
- Denicolò S, Vogi V, Keller F, Thöni S, Eder S, Heerspink HJL, Rosivall L, Wiecek A, Mark PB, Perco P, Leierer J, Kronbichler A, Steger M, Schwendinger S, Zschocke J, Mayer G, Jukic E. Clonal Hematopoiesis of Indeterminate Potential and Diabetic Kidney Disease: A Nested Case-Control Study. Kidney Int Rep. 2022 Feb 3;7(4):876-888. doi: 10.1016/j.ekir.2022.01.1064. PMID: 35497780; PMCID: PMC9039487.
Selection of Funding
- FWF P33333 to Markus Keller
- FWF P34574 to Markus Keller
- FWF I 6477-B to Katharina Wimmer
- FFG 878654 to Markus Keller
- DOC-Fellowship (ÖAW) to Jakob Koch
- DOC-Fellowship (ÖAW) to Yvonne Wohlfarter
- Legerlotz Stiftung to Yvonne Wohlfarter
Collaborations
- Daltro Cardoso Luiz Helena, Oroboros Instruments, Innsbruck, Austria
- Gnaiger Erich, Oroboros Instruments, Innsbruck, Austria
- Hauke S. Hillen, Department of Cellular Biochemistry, University Medical Center Göttingen, Göttingen, Germany & Research Group Structure and Function of Molecular Machines, Max Planck Institute for Multidisciplinary Sciences, Göttingen, Germany & Cluster of Excellence ‘Multiscale Bioimaging: from Molecular Machines to Networks of Excitable Cells’ (MBExC), University of Göttingen, Göttingen, Germany
- Ryan Yu, Department of Cellular Biochemistry, University Medical Center Göttingen, Göttingen, Germany & Research Group Structure and Function of Molecular Machines, Max Planck Institute for Multidisciplinary Sciences, Göttingen, Germany
- Richard Gallon, Cancer Prevention Research Group, Translational and Clinical Research Institute, Faculty of Medical Sciences, Newcastle University, Newcastle, UK
- Thielens, Nicole, Gaboriaud, Christine Univ. Grenoble Alpes, CEA, CNRS, Institut de Biologie Structurale (IBS), Grenoble, France.