Müllerstraße 59
6020 Innsbruck
Fax: +43 (0)512 9003 71112
Email: Marko.Konschake@i-med.ac.at
Website: https://www.i-med.ac.at/Anatomie/
Research year
Research Branch (ÖSTAT Classification)
301102, 301103, 301104, 301106, 301107, 301111, 301112, 301113
Keywords
anatomical collection, Clinical-applied anatomy, digitalisation, human development, immunohistochemistry, lymphology, neuromonitoring, peripheral nerves, pre-/postgraduate education, and ultrasound
Research Focus
Our Institute has two major research topics: clinically applied anatomy and human development. We are applying classical anatomical methods in combination with modern imaging tools and operative and biomechanical methods. Human development is an important mainstay for descriptive and modern clinical and functional anatomy. We are microscopically examining various human adult and embryonic samples. 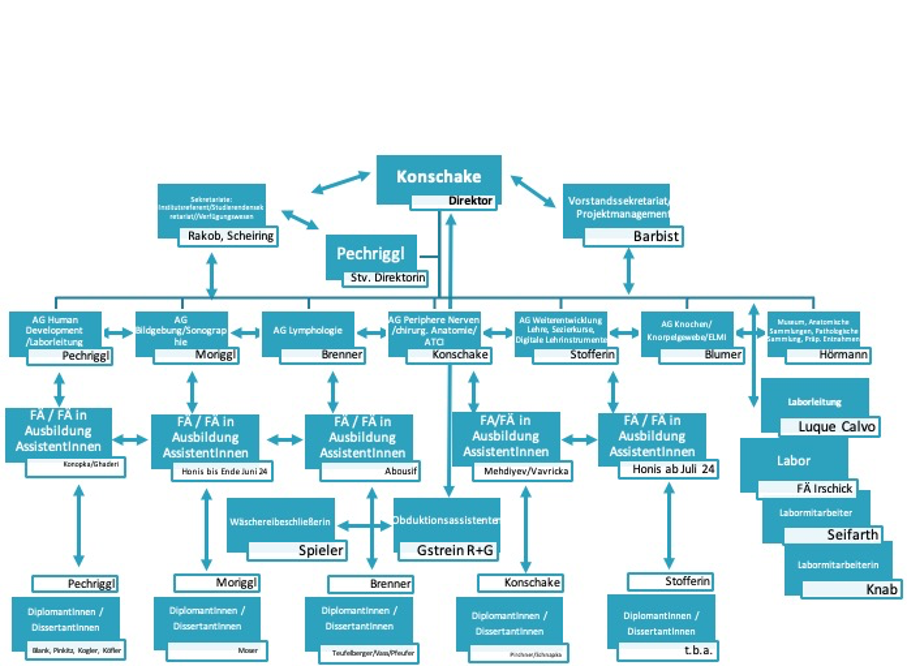
General Facts
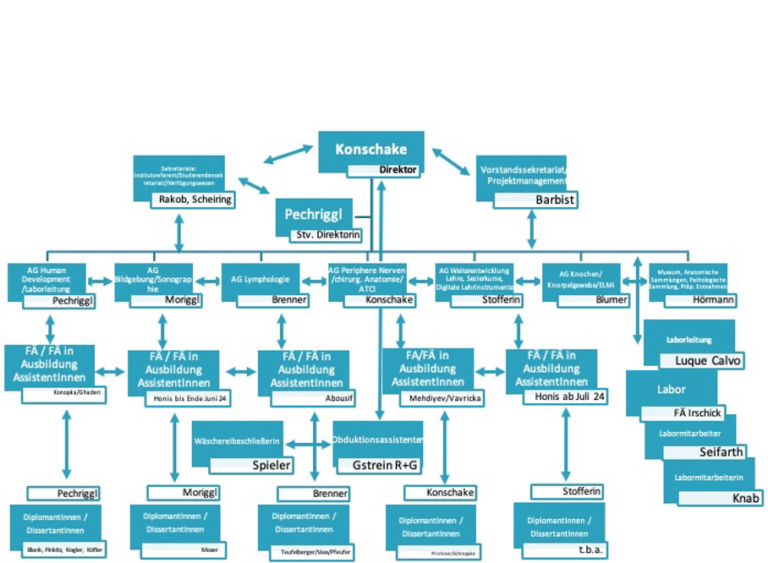
The major tasks of our Institute are research and education in human anatomy and embryology. The aim of anatomy is to understand the normal structures and architecture of the organism, while embryology aims to understand its normal development. Our research and education rely on an extensive body donation programme. We offer numerous lectures and pre- and postgraduate courses in anatomy for MDs, medical students, PhD students and physicians of various medical disciplines. We have seven research groups, all of which follow a translational, clinically applied approach, with an emphasis on peripheral nerves, human development, imaging, lymphology, skeletal development/ELMI, education and digitization and anatomical collection. We are applying a range of anatomical techniques in collaboration with national and international universities and university hospitals.
The graphic illustrates our scientific working groups (and personal team workflow):
Research
AGs Peripheral Nerves/Surgical Anatomy/ATCI and Imaging/Sonography (Konschake,Moriggl)
Our “Applied Anatomy” research is creating new minimally invasive surgical techniques, enhancing modern imaging methods and developing new clinical applications, particularly in neuro-sonography, using body donors and volunteers to ensure immediate implementation in patient care. The research is relevant to areas such as interventional radiology, (plastic) surgery and ENT, as well as to regional anaesthesia and pain management.
Common complications following low anterior resections include urinary and faecal incontinence and sexual dysfunction, often linked to damage of the pelvic autonomic nerves during surgery. To help surgeons protect these nerves, we have explored a new method for intraoperative pelvic neuromonitoring that relies on impedance measurements of the innervated organs. We have developed an algorithm (named AMINA) to classify bioimpedance signals. Thirty patients participated in a clinical trial involving nerve-preserving robotic rectal surgery with intraoperative pelvic neuromonitoring. The contraction of the urinary bladder and/or rectum, induced by direct stimulation of the nerves, led to a change in tissue impedance signals. We calculated features of the impedance signal and classified them to form AMINA, which automates the interpretation of impedance signals during intraoperative pelvic neuromonitoring. (Fig.1.)
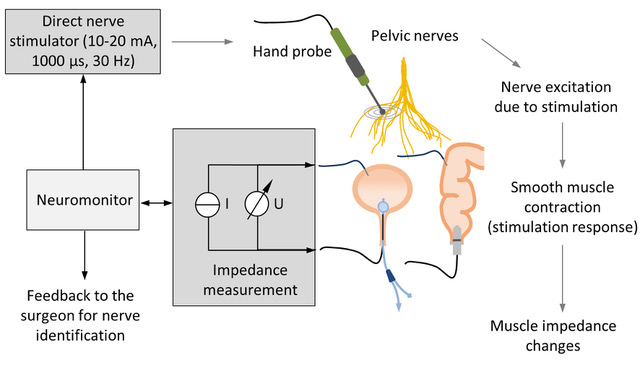
bioimpedance measurement of the bladder and rectum with a bipolar electrode montage. Contraction of the bladder and rectum smooth muscle in response to direct stimulation of the innervating autonomic nerves results in a change in the muscle tissue impedance. Assessment of stimulation-induced changes in tissue impedance allows the surgeon to identify and preserve the nerves. © Scientific Reports
Another example of our translational approach is “carpal tunnel release”, a common procedure with a high success rate but with the risk of iatrogenic injuries. We have explored the anatomy of the carpal tunnel, focusing on the proximity of neurovascular structures prone to injury, particularly during minimally invasive procedures. We noted the anatomical positions of the median nerve, ulnar nerve, ulnar artery and Berrettini branch in the carpal tunnels of 104 wrists from 52 body donors. We identified four major anatomical risk situations: (1) a proximal division of the middle-finger/ring-finger branch of the median nerve with a narrow safe zone; (2) an ulnar take-off of the recurrent muscle branch of the median nerve close to the distal ulnar end of the carpal tunnel; (3) an ulnar artery arch near the transverse carpal ligament; and (4) a proximal Berrettini branch close to this ligament. These anatomical features may place some patients at a higher risk of neurovascular injuries during minimally invasive carpal tunnel releases. We recommend a preoperative ultrasound assessment of at-risk neurovascular structures for those undergoing endoscopic or ultrasound-guided tunnel release. In high-risk patients, open surgery may be the safer option.
AG Human Development (Pechriggl)
VasKo: Vascular senescence as a key factor for cochlear health
Age-related hearing loss (ARHL) affects approximately one third of people over 65 worldwide. Both neuronal and vascular factors have a crucial role in its pathophysiology. We are investigating the impact of vascular senescence on cochlear health and developing new therapeutic approaches. We believe that age-related changes in cochlear blood supply impair the function of spiral ganglion neurons and thereby limit the success of cochlear implants (CI) in elderly patients. Improving cochlear blood flow by electrical stimulation (ES) or local pharmacological intervention could preserve neuronal vitality and optimize CI performance. This therapeutic approach requires a thorough assessment of age-related vascular alterations in the cochlea. We are characterizing the vascular microarchitecture of post-mortem human cochleae of 30 body donors by micro-CT and histological techniques. We are also examining the internal carotid artery to assess vascular health.
The project is being performed in cooperation with Med-El. It will provide the first systematic mapping of the cochlear vasculature in the geriatric population and will identify potential therapeutic targets. In the long term, we may even be able to modulate cochlear blood flow by implanted electrodes.
AG Lymphology – Phlebology (Brenner)
Our major accomplishment was the publication of the book ‘Angewandte Lymphologie’ (together with Manuel E. Cornely and Wolfgang C. Marsch) in 2023 (Cornely et al., 2023; Figure 1); an English edition is due in 2025. Several papers have dealt with so-called lipoedema (Brenner, 2023; Brenner et al., 2023): we showed that body mass index is not a valid measure for the diagnosis of lipoedema and should be replaced by waist-to-height ratio. We also created a new S2k guideline on this clinical picture (Faerber et al., 2024; Figure 2). We have worked on the effectiveness of manual lymphatic drainage in breast cancer-related lymphedema (Kasseroller and Brenner, 2023) and Erich Brenner is involved in the development of an S3 guideline on the diagnosis and treatment of lymphoedema (AWMF Reg. No. 058-001), which is funded by the GBA Innovation Fund.
AG Skeletal Development/ELMI (Blumer)
Structural colouration in nature
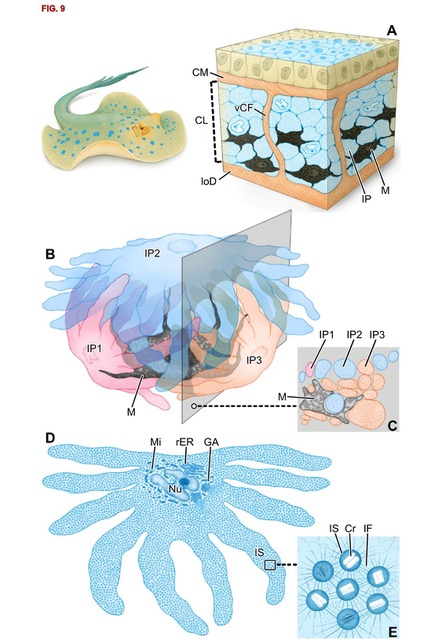
In animals, pigments and nanostructures determine skin colouration. We recently discovered a new mechanism for generating the blue colouration in the ribbontail stingray Taeniura lymma, a species with electric blue spots on its yellow-brown skin. We characterized fine-scaled differences in cell composition and architecture that distinguish blue from non-blue regions. In blue regions, the upper dermis comprises a layer of chromatophore units, iridophores and melanophores entwined in compact clusters framed by collagen bundles. Stingray iridophores differ from other vertebrate light-reflecting cells in having numerous finger-like processes that surround nearby melanophores like fists clenching a black stone. Iridophores contain spherical iridosomes that enclose guanine nanocrystals, suspended in a 3D quasi-order, linked by a cytoskeleton of intermediate filaments. We argue that intermediate filaments form a structural scaffold with a distinct optical role, providing the iridosome spacing critical to produce the blue colour. In contrast, black-pigmented melanosomes within melanophores show space-efficient packing, consistent with their hypothesized role as broadband absorbers for enhancing the blue colour. In non-blue areas, iridophores are replaced by pale cells, resembling iridophores in some morphological and nanoscale features but lacking guanine crystals. The cellular associations and structural interactions in stingray skin suggest that pigment cells induce differentiation in the progenitor cells of iridophores and that some features driving colour production may be shared with bony fishes, although the lineages diverged hundreds of millions of years ago and the iridophores themselves differ drastically. (Fig. 3)
Pictures
Selected Publications
Kaiser P, Schmidle G, Bode S, Seeher U, Honis HR, Moriggl B, Pechriggl E, Stofferin H, Konschake M. Clinical-applied anatomy of the carpal tunnel regarding mini-invasive carpal tunnel release. Arch Orthop Trauma Surg. 2024 Nov;144(11):4753-4765. https://doi.org/10.1007/s00402-024-05560-7
Schuler R, Langer A, Marquardt C, Kalev G, Meisinger M, Bandura J, Schiedeck T, Goos M, Vette A, Konschake M. Automatic muscle impedance and nerve analyzer (AMINA) as a novel approach for classifying bioimpedance signals in intraoperative pelvic neuromonitoring. Sci Rep. 2024 Jan 5;14(1):654. https://doi.org/10.1038/s41598-023-50504-7
Brenner, E., Forner-Cordero, I., Faerber, G., Rapprich, S., Cornely, M., 2023. Body mass index vs. waist-to-height-ratio in patients with lipohyperplasia dolorosa (vulgo lipedema). J Dtsch Dermatol Ges 21:1179-1185. http://dx.doi.org/10.1111/ddg.15182.
Surapaneni, V.A., Blumer, M.J., Tadayon, K., McIvor, A.J., Redl, S., Hornis, H.-R., Mollen, F.H., Amini, S., Dean, M.N. (2024). Ribbontail Stingray Skin Employs a Core–Shell Photonic Glass Ultrastructure to Make Blue Structural Color. Adv. Optical Mater. https://doi.org/10.1002/adom.202301909
Selection of Funding
- FFG Bridge Grant: KFA-Medel
- FEI Grant: KFA-EyeCre-Addion “Forschung Entwicklung Innovation” Land Tirol, F.47167/9-2023
- Educational Grants KFA-Medel
Collaborations
Prof.Dr. José Sanudo, MD, Prof.in Dr.in Teresa Vazquez, MD, Complutense University of Madrid, Center of Body Donation, Madrid, Spain
Prof.Dr. Jeffrey Janis, MD, FACS, Department of Plastic Surgery, Ohio State University Medical Center in Columbus, Ohio, USA
Univ.Prof.Dr. Fabrice Duparc, Université de Rouen, Rouen, France
Prof.Dr. David Chen, MD, FACS, Lichtenstein Amid Hernia Clinic at UCLA Section of Minimally Invasive Surgery, UCLA Division of General Surgery, Los Angeles, USA
Univ.-Prof. Thomas Fichtner-Bendtsen, MD, Ass.-Prof. Jens Børglum Neimann, MD, Department of Clinical Medicine Anesthesiology, Copenhagen, Denmark
Univ.-Prof. Vincent Chan, MD, University Health Network – Toronto Western Hospital, Toronto, Canada
Department of Infectious Diseases & Public Health Jockey Club College of Veterinary Medicine and Life Sciences City University of Hong Kong, Department of Biomaterials Max Planck Institute of Colloids and Interfaces (Germany).