Innrain 66
6020 Innsbruck
Fax: +43 (0)50 504 27390
Email: georg.dechant@i-med.ac.at
Website: https://www.i-med.ac.at/ge_nw/
Research year
Research Branch (ÖSTAT Classification)
106023, 106039, 301114, 301401, 301402, 304003
Keywords
cognition, functional genomics, intelligence, molecular psychiatry, neuroepigenetics, neurogenomics, and neuroplasticity
Research Focus
A considerable number of cognitive impairments observed in conditions such as Alzheimer’s disease, schizophrenia, autism spectrum disorders, and intellectual disabilities are the result of disruptions in molecular pathways. Our genomic and transcriptomic studies reveal how dynamic changes in the 3D chromatin structure of neuronal nuclei influence cognition. A comprehensive molecular understanding paves the way for the development of pharmaceuticals or other inventions aimed at enhancing memory, focus or learning in both clinical and non-clinical populations.
General Facts
The Institute for Neuroscience is a small, dynamic research unit comprising four scientists, namely Univ. Prof. Georg Dechant, Assist. Prof. Galina Apostolova, Dr. Nico Wahl and Dr. Mujahid Ali, in addition to PhD and master students. The Institute provides access to cutting-edge infrastructure and sophisticated research equipment. The institution’s core strengths are centred on three key areas: functional genomics, comprehensive bioinformatics analyses, the generation and analysis of transgenic mouse models as well as the neuronal differentiation of human induced pluripotent stem cells (iPSCs). We serve as past and current principal investigators in several FWF-funded research networks on campus, including:
- The doctoral school Signal Processing in Neurons (SPIN) (www.neurospin.at)
- The SFB-F44 Cell Signaling in Chronic CNS Disorders (link)
- The recently launched Cluster of Excellence CO16 Neuronal Circuits in Health and Disease
It is also noteworthy that the present institution collaborates closely with leading experts in the field of 3D chromatin analysis (e.g., S. Akbarian, Mount Sinai, New York; A. Pombo, MDC
Berlin), and human genetics (D. Morris, Galway). These collaborative endeavours have culminated in the acquisition of a comprehensive array of neuroscience technologies.
The role of Assistant is to provide support and assistance to the relevant parties. Assist. Prof. Galina Apostolova and Dr. Nico Wahl are also deeply engaged with the SATB2 research and patient community. They fulfil the role of scientific advisors for SATB2 Europe, a patient-driven initiative that aims to enhance the quality of life for individuals diagnosed with SATB2-associated syndrome (satb2europe.org).
Research
The present study combines mouse and human stem cell models of psychiatric disorders with systems biology and functional genomics in order to elucidate the mechanisms affecting cognition. The objective of this study is to identify novel molecular targets within the neuronal nucleus. The present research is focused on the influence of chromatin architecture in neurons on cognitive processes.
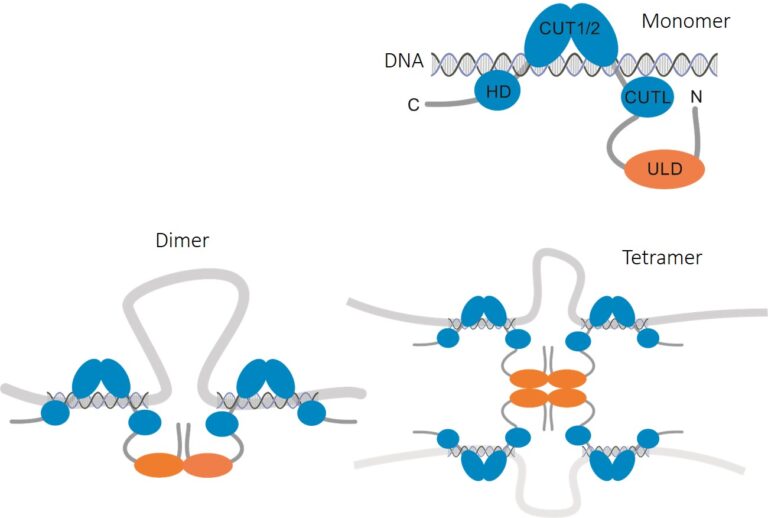
A key focus of our research is Special AT-rich Sequence-Binding Protein 2 (SATB2), a highly conserved, vertebrate-specific DNA-binding protein. Spontaneous mutations in the SATB2 gene have been identified as the underlying cause of SATB2-associated syndrome (SAS; OMIM 612313), an autosomal dominant neurodevelopmental disorder characterised by severe intellectual disability and limited to absent speech. Furthermore, common genetic variation in SATB2 has been associated with an elevated risk of developing schizophrenia, autism spectrum disorders and inter-individual differences in general cognitive ability.
In collaboration with D. Morris (National University of Ireland), our institute has demonstrated that SATB2 target genes, in addition to genes encoding SATB2-interacting proteins, are among the most constrained genes in the human genome. The present studies demonstrate that rare, high-impact mutations in these genes are the causative agents of severe cognitive disorders. In contrast, common, low-impact variants have been shown to modulate cognitive performance in the general population, to contribute to susceptibility for schizophrenia and to influence educational attainment (Whitton et al., 2018; Cera et al., 2019).
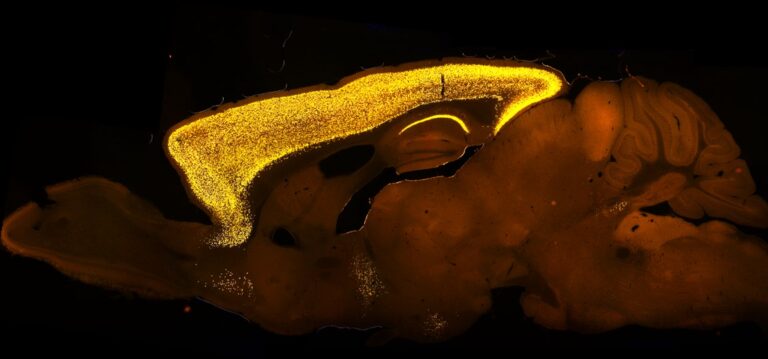
Within the Central Nervous System (CNS), SATB2 displays a highly selective and dynamic expression pattern in the forebrain, consistent with its proposed role in cognition
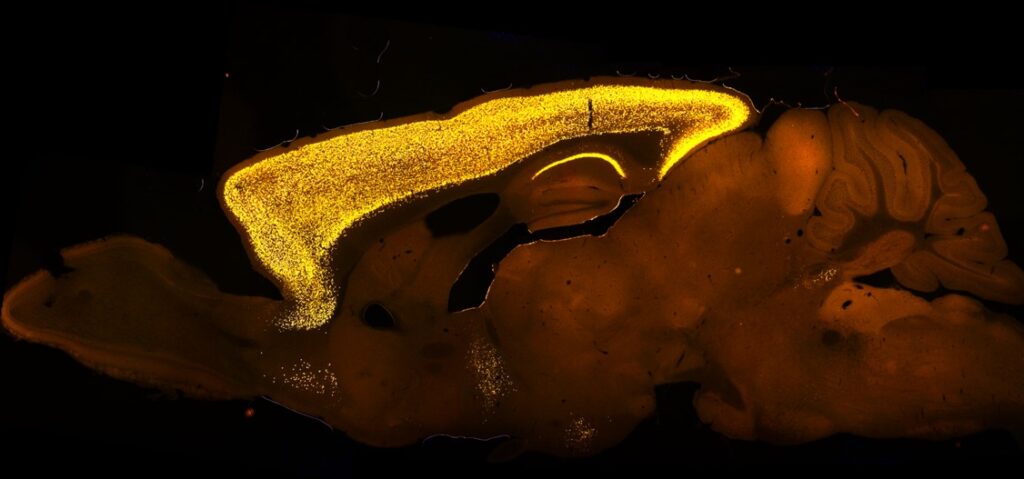
As demonstrated in previous studies, SATB2 is imperative for the consolidation of long-term memory, Schaffer collateral long-term potentiation (LTP), and the regulation of activity-dependent gene expression in pyramidal neurons (Cera et al., 2019; Feurle et al., 2021; Jaitner et al., 2016).
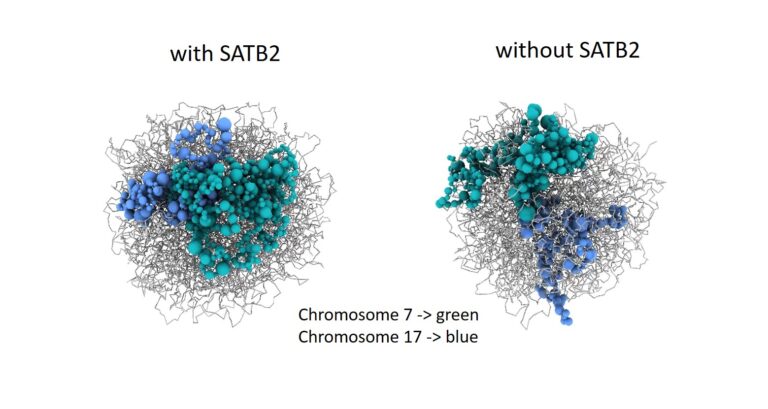
Spatial genome organisation, including interactions between chromatin and the nuclear lamina (NL), has been identified as a crucial regulatory layer for gene expression (Winick-Ng et al., 2021). to the hypothesis that SATB2 modulates 3D chromatin structure through homomeric DNA binding has been postulated. In our recent publication (Wahl et al., 2024), we tested this hypothesis in collaboration with S. Akbarian (Icahn School of Medicine, Mount Sinai, New York) and investigated SATB2’s role in shaping the 3D genome and chromatin accessibility in cortical neurons. The present findings demonstrate that SATB2 is involved in the process of chromatin looping between enhancers and promoters of activity-regulated genes, thereby enabling their transcription. Furthermore, SATB2 influences both intra- and inter-chromosomal interactions
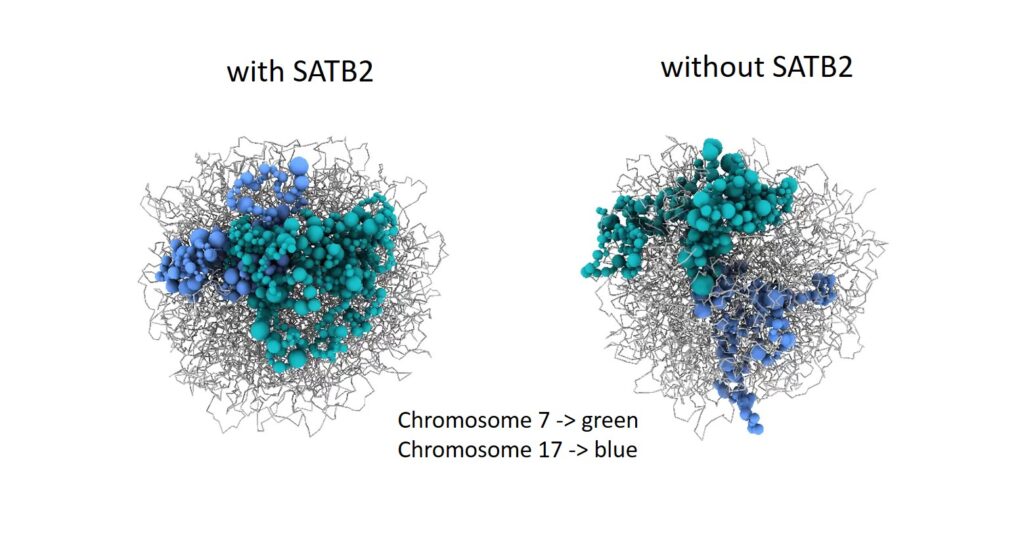
Genes that are subject to the effects of SATB2-dependent 3D genome remodelling are found to be strongly associated with specialised neuronal functions, cognitive performance, and vulnerability to neurodevelopmental and neuropsychiatric disorders. The data obtained has positioned SATB2 as a pivotal, cell type-specific regulator of the 3D genome, with the capacity to shape the structure and expression of approximately 50% of all genes associated with cognition.
We are currently extending this work by studying SATB2 function in human iPSC-derived cortical neurons. These analyses identify the evolutionarily conserved and human-specific roles of SATB2.
We are also investigating whether activity-dependent remodelling of 3D chromatin architecture in pyramidal neurons requires SATB2. Finally, we hypothesise that SATB2 expression in juvenile neurons is vital for converting sensory experiences and stress exposure into long-lasting, gene regulatory chromatin configurations. These are the molecular imprints that are critical for adult cognitive function and that may underlie risk for neuropsychiatric disease.
Cera I, Whitton L, Donohoe G, Morris DW, Dechant G*, Apostolova G* Genes encoding SATB2-interacting proteins in adult cerebral cortex contribute to human cognitive ability (*equal contribution). PLoS Genet. 2019 Feb 6;15(2)
Feurle P, Cera I, Abentung A, Wahl N, Ablinger C, Bucher M, Stefan E, Sprenger S, Teis D., Fischer A, Laighneach A, Whitton L, Morris DW, Apostolova G*and Dechant G*. SATB2-LEMD2 interaction links plasticity in nuclear shape to gene regulation underpinning cognition (*equal contribution), EMBO J. 2021 Feb 1;40(3):e103701
Jaitner C, Reddy C, Abentung A, Whittle N, Rieder D, Delekate A, Korte M, Jain G, Fischer A, Sananbenesi F, Cera I, Singewald N, Dechant G* and Apostolova G*. Satb2 Regulates miRNA Expression and Long-Term Memory in Adult CNS. (*equal contribution) eLife.2016;5:e17361. DOI: 10.7554/ 17361.
Wahl N, Espeso-Gil S, Chietera P, Nagel A, Laighneach A, Morris DW, Rajarajan P, Akbarian S, Dechant G*, Apostolova G* SATB2 organizes the 3D genome architecture of cognition in cortical neurons. (*equal contribution), Mol Cell. 2024 Feb 15;84(4):621-639.e9. doi: 10.1016/j.molcel.2023.12.024. Epub 2024 Jan 19.
Winick-Ng W, Kukalev A, Harabula I, Zea Redondo L, Szabo D, Meijer M, Serebreni L, Zhang Y, Bianco S, Chiariello AM, Irastorza-Azcarate I, Fiorillo , Musella F, Thieme CJ, Irani E, Torlai Triglia E, Kolodziejczyk AA, Abentung A, Apostolova G, Paul EJ, Franke V, Kempfer R, Akalin A, Teichmann SA, Dechant G, Ungless MA, Nicodemi M, Welch L , Castelo-Branco G and Pombo A. Cell-type specialization in the brain is encoded by specific long-range chromatin topologies. Nature. 2021 Nov;599(7886):684-691
Whitton I, Apostolova G, Dechant G, Rieder D, Rea S, Donohoe G and Morris D. Genes involved in neurodevelopmental processes and regulated by SATB2 contribute to schizophrenia and cognition PLoS Genet. 2018:e1007515
Pictures
Selected Publications
Development and evolution of Drosophila chromatin landscape in a 3D genome context.
Ali M, Younas L, Liu J, He H, Zhang X, Zhou Q. Nat Commun. 2024 Nov 1;15(1):9452
SATB2 organizes the 3D genome architecture of cognition in cortical neurons.
Wahl N, Espeso-Gil S, Chietera P, Nagel A, Laighneach A, Morris DW, Rajarajan P, Akbarian S, Dechant G*, Apostolova G* (equal contribution). Mol Cell. 2024 Feb 15;84(4):621-639.e9.
Skeletal muscle transcriptomics dissects the pathogenesis of Friedreich’s ataxia.
Indelicato E, Kirchmair A, Amprosi M, Steixner S, Nachbauer W, Eigentler A, Wahl N, Apostolova G, Krogsdam A, Schneider R, Wanschitz J, Trajanoski Z, Boesch S. Hum Mol Genet. 2023 Jun 19;32(13):2241-2250.
Selection of Funding
Georg Dechant
FWF P 32850 SATB2 abhängige Mechanismen und Kognition
Euro 400.172,-
15.1.2020 – 14.7.2024
FWF P 35584 SATB2 reguliert kritische Perioden und Stressresilienz
Euro 424.307,-
1.10.2022 – 30.9.2026
Galina Apostolova
FWF P33027 SATB2-Regulation der neuronalen Zellkernarchitektur
Euro 411.952,-
19.10.2020 – 18.4.2025
FWF Cluster of Excellence CO16 ‘Neuronal circuits in health and disease’
TBD
Nico Wahl
Tiroler Wissenschaftsfond (TWF) SATB2-dependent regulation of cognition related genes
Euro 37.822,-
1.11.2024 – 30.11.2026
Collaborations
Schahram Akbarian, MD, PhD
Division of Psychiatric Epigenomics, Departments of Psychiatry and Neuroscience, Mount Sinai School of Medicine, New York
Derek Morris, PhD
Centre for Neuroimaging and Cognitive Genomics (NICOG) at National University of Ireland (NUI).
Prof. Dr. Ana Pombo
Max-Delbrück-Centrum für Molekulare Medizin (MDC) Berlin, Deutschland