Anichstraße 35
6020 Innsbruck
Fax: +43 512 504 23002
Email: matthias.schmuth@i-med.ac.at
Website: http://dermatologie.tirol-kliniken.at/
Research Branch (ÖSTAT Classification)
302011, 301902, 301904, 302002, 302087, 302055
Keywords
allergy, cancer, Cell biology, dendritic cells, Immunology, Inflammation, Keratinocytes, and melanoma
Research Focus
In the dermatology department, basic research and patient care are intimately interconnected. This will ultimately be advantageous to our patients, who will be able to benefit from new diagnostic and therapeutic approaches early in development. Our interconnected research program covers tumour biology, the cutaneous immune system, allergies, autoimmunity, infectious diseases of the skin, HIV infection, genetic skin diseases and dermatohistopathology.
General Facts
The department is one of the oldest and internationally most renowned dermatology departments in the German-speaking countries. Approximately 3,000 inpatients and 50,000 outpatients are seen every year.
The department focuses on complex dermatology and has a strong track record in translational research.
Within the main research areas of the university, the department conducts research programmes in the areas of: 1) infection immunity and transplantation; 2) oncology; 3) genetics, epigenetics and genomics; 4) inflammation including translational research on the most common chronic inflammatory skin disease worldwide namely atopic dermatitis.
- HIV patients are studied in a national OEHIVKOS cohort, which was initiated in and is run from Innsbruck, and which is well connected with international registries. Novel treatments for allergies are developed within the apple care consortium (EU Interreg).
- The department is certified both by Quality Austria (ISO 9001) and by OnkoZert (German Cancer Society). Research programmes are coordinated within the Austrian oncology group (ÖGDV) and the melanoma consortium MEMS (EU Interreg).
- The department is designated as a centre of expertise (Type B centre) and European Reference Network (ERN) for genodermatoses with a focus on disorders of cornification. Its research focuses on discovering novel mutations in genodermatoses and on developing gene and antibody-mediated therapies.
Research
Apple Care
Norbert Reider and Bettina Nothegger
Most patients who are allergic to birch pollen develop allergic cross-reactions to the major allergen found in apples, Mal d1, known as pollen-related food allergy (prFA). This is due to a strong, clinically relevant homology between the major allergen in birch, Bet v 1, and Mal d 1. Daily apple consumption induces oral tolerance in prFA, but its effect on inhalational allergy has not been investigated. This study aimed to uncover apple cultivars that are suitable for the treatment of birch pollen rhinoconjunctivitis and apple allergy in a controlled and established dosage. From this testing, we provide a ranking of 23 cultivars. Skin reactivity increased from flesh to peel to stalk, and SPT results were able to predict the severity of prFA for each allergenicity class. We are currently developing a treatment protocol for allergen immunotherapy to birch pollen and prFA with daily apple consumption.
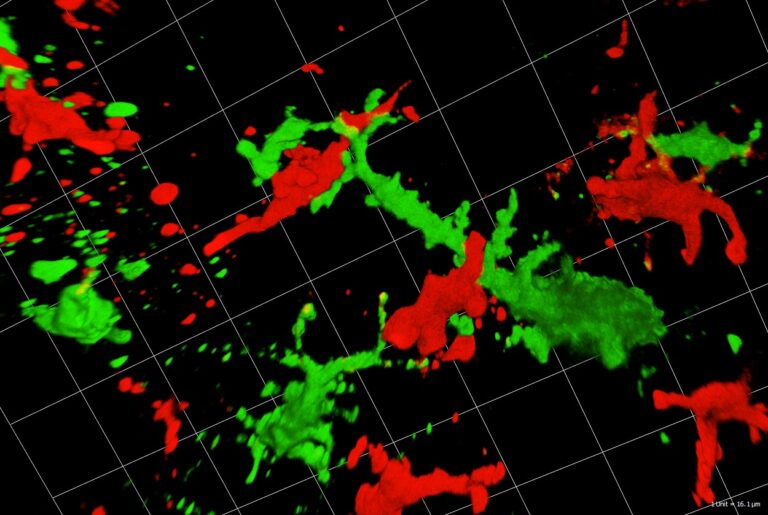
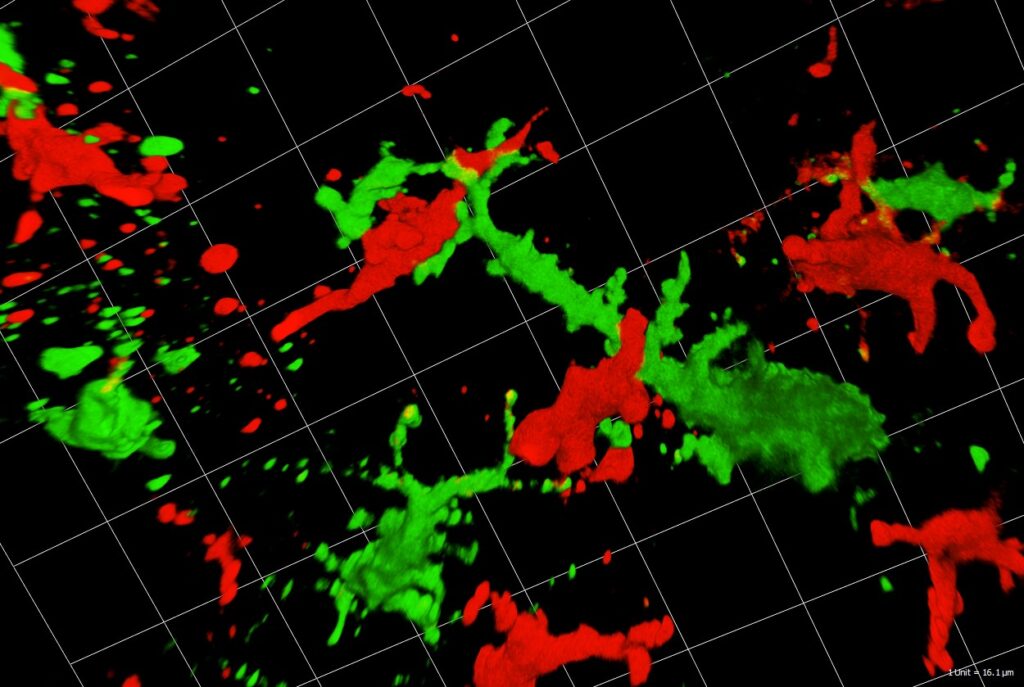
Immunosurveillance of Skin by Dendritic Cells – Potential Application for Immunotherapy
Patrizia Stoitzner, Christoph Tripp, Florian Hornsteiner, Claudia Zelle-Rieser, Helen Strandt, Sophie Dieckmann, Janine Vierthaler, Christina Mayerl
Dendritic cells (DC) act as sentinels of the skin immune system and initiate humoral and cellular immunity. This unique ability renders them optimal targets for immunotherapy of various skin diseases, such as melanoma. Indeed, DC-based immunotherapy has proven its potential to prime T cell responses against tumours but clinical responses are insufficient. To improve DC-based immunotherapy, we need to understand how skin DC are affected by tumour development. We are specifically interested in understanding the effects of altered metabolism and infiltration of suppressive immune cells on DC function. With the help of mouse models for melanoma, we investigate skin DC subsets in tumours both phenotypically and functionally by means of multiplex flow cytometry and microscopy as well as immunoassays. With this knowledge, we can further develop skin immunisation strategies for the treatment of cancer. At present, we are working on targeting antigens to skin DC subsets with the help of antibodies against C-type lectin receptors DEC-205 and Langerin in mouse models, but also human skin explant cultures. Moreover, we are testing a novel immunotherapeutic approach using nanoparticles coated with a specific Langerin ligand. As the future of cancer treatment lies in combination therapy, we are evaluating the potential of using DC-based immunotherapy together with tumour-targeted therapy, e.g. BRAF/MEK-inhibitor therapy. In collaboration with the 3D-biorpinting lab at the MUI we are also developing future alternatives for animal experiments, such as immunocompetent 3D-bioprinted skin/melanoma-on chip-models. These novel models will allow in situ investigation by live cell imaging of DC interacting with tumour cells during tumour-targeted therapy.
These diverse model systems will enable us to unravel communication between DC subtypes and tumour cells with the ultimate goal to advance existing therapeutic strategies.
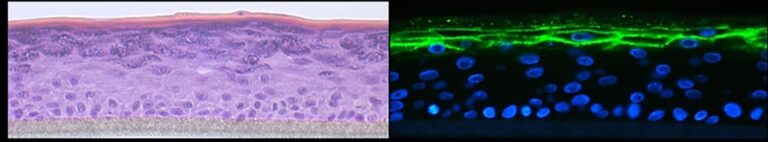
Atopic Dermatitis
Sandrine Dubrac, Deborah Minzaghi, Verena Moosbrugger-Martinz
Atopic dermatitis is the most common chronic, inflammatory skin disease worldwide with a yearly prevalence of 1-23% in both children and adults. It is characterised by Th2 inflammation over dry skin. Moreover, atopic dermatitis is associated with comorbidities, which lead to a significant disease burden on patients and societies. Our research is dedicated to a better understanding of the pathogenesis of atopic dermatitis by using a holistic approach including metabolic abnormalities, skin microbiota and systemic immune abnormalities. An essential tool of our research is high-quality 3D organotypic cultures that we generate with patients’ keratinocytes. In addition, we have demonstrated that Langerhans cells are key players in the development of atopic dermatitis. Our recent research has breached the paradigm that all patients with atopic dermatitis are superinfected with staphylococcus aureus. What’s more, we were the first to decipher the role of the pregnane x receptor (PXR), a master regulator of xenobiotic metabolism, in the skin by demonstrating that PXR overexpression leads to atopic dermatitis–like symptoms. Interestingly, our recent research has pointed to a major role of PXR in the skin response to environmental pollutants such as endocrine disruptors.With in-house dermatologists, we are currently deciphering the molecular pathomechanisms behind the comorbidities in atopic dermatitis. This research is largely empowered by a tight collaboration with the Department of Human Genetic (MUI). Last but not least, we strive to develop novel therapeutic strategies to treat inflammatory skin diseases (patent EP22187107.2) in the context of a translational research project.
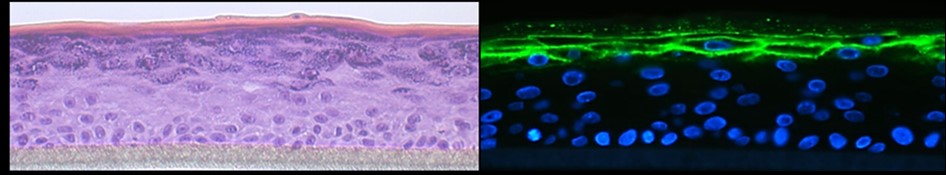
Gene Therapy
Daniela Ortner-Tobider, Thomas Trafoier, Matthias Schmuth, Christina Heufler-Tiefenthaler
The goal of this project is to correct keratin filament fragility by means of gene editing approaches (CRISP/Cas9), to ameliorate disorders of cornification.
Our aim is to correct the intermediate filament fragility in EPPK patient-derived keratinocyte (KC) cultures with keratin 9 mutations by means of CRISPR/Cas9-mediated deletion of the mutant, disease-causing allele whilst leaving the wild-type allele intact. We targeted a gRNA pair complex to Cas9 nickase in primary, patient-derived KCs and we grew single clones from the transfected bulk population. Genetic characterisation of these clones revealed a collection of clones with the wild-type allele intact and the mutated allele truncated. We are now aiming at delivering this repair strategy to skin cells in vivo to translate our basic science findings into the clinic.
Epidermal Homeostasis and Genetic Skin Diseases
Robert Gruber, Stefan Blunder, Verena Moosbrugger-Martinz, Matthias Schmuth
This research group focuses on the regulation of homeostasis and skin barrier function, as exemplified in rare ichthyoses as a result of defined gene mutation.
Cutaneous Immune System, Autoimmunity
Barbara Böckle, Gudrun Ratzinger
This group has established a registry of autoimmune skin disorders. Clinical and immune parameters are included with information about response to therapy and they therefore provide a rich scientific resource to address questions both of disease mechanisms and of therapeutic strategies.
Infectious Diseases of the Skin
Robert Zangerle, Mario Sarcletti, Martin Gisinger, Maria Kitchen-Hosp
This programme addresses questions of HIV epidemiology and response to therapy using the national OEHIVKOS cohort, which is linked to international collaborative efforts, e.g. the Antiretroviral Therapy Cohort Collaboration (ART-CC), the Collaboration of Observational HIV Epidemiological Research Europe (COHERE) in EuroCoord, the CASCADE Collaboration in EuroCoord, and EuroSIDA in EuroCoord.
Photomedicine
Gudrun Ratzinger
This programme addresses the effects of UV irradiation as a causative factor in photodermatoses as well as the therapeutic effects of UV irradiation on common inflammatory skin diseases and skin cancer. Additionally, we participate in the Austrian Psoriasis Registry (PsoRA), in order to gain clinical and epidemiological data.
Dermatohistopathology
Nina Frischhut
Dermatohistopathology research in Innsbruck is renowned for the innovative concepts that describe a variety of morphological discoveries in skin disorders.
Clinical Trials
Norbert Reider, Gudrun Ratzinger, Mario Sarcletti, Van Anh Nguyen, Georg Weinlich, Robert W. Gruber, Matthias Schmuth
The department’s clinical trial unit conducts numerous phase I – III clinical trials on chronic inflammatory skin disease (psoriasis), allergies, skin cancer (principally melanoma), HIV and genetic skin diseases.
Pictures
Selected Publications
- Probst HC, Stoitzner P, Amon L, Backer RA, Brand A, Chen J, Clausen BE, Dieckmann S, Dudziak D, Heger L, Hodapp K, Hornsteiner F, Hovav AH, Jacobi L, Ji X, Kamenjarin N, Lahl K, Lahmar I, Lakus J, Lehmann CHK, Ortner D, Picard M, Roberti MP, Rossnagel L, Saba Y, Schalla C, Schlitzer A, Schraml BU, Schütze K, Seichter A, Seré K, Seretis A, Sopper S, Strandt H, Sykora MM, Theobald H, Tripp CH, Zitvogel L.Guidelines for DC preparation and flow cytometry analysis of mouse nonlymphoid tissues. Eur J Immunol. 2023 Nov;53(11):e2249819. doi: 10.1002/eji.202249819. PMID: 36512638. Probst and Stoitzner both lead authors.
- Unterhauser J, Ahammer L, Rainer T, Eidelpes R, Führer S, Nothegger B, Covaciu CE, Cova V, Kamenik AS, Liedl KR, Müller T, Breuker K, Eisendle K, Reider N, Letschka T, Tollinger M. Covalent polyphenol modification of a reactive cysteine in the major apple allergen Mal d 1. Food Chem. 2023 Jun 1;410:135374. doi: 10.1016/j.foodchem.2022.135374. PMID: 36608553
- Jaschinski N, Greenberg L, Neesgaard B, Miró JM, Grabmeier-Pfistershammer K, Wandeler G, Smith C, De Wit S, Wit F, Pelchen-Matthews A, Mussini C, Castagna A, Pradier C, d’Arminio Monforte A, Vehreschild J, Sönnerborg A, Anne AV, Carr A, Bansi-Matharu L, Lundgren J, Garges H, Rogatto F, Zangerle R, Günthard HF, Rasmussen LD, Nescoi C, Van Der Valk M, Menozzi M, Muccini C, Mocroft A, Peters L, Ryom L; RESPOND Study Group. Recent abacavir use and incident cardiovascular disease in contemporary-treated people with HIV. 2023 Mar 1;37(3):467-475. doi: 10.1097/QAD.0000000000003373. PMID: 36001525
- Hornsteiner F, Sykora MM, Tripp CH, Sopper S, Stoitzner P. Mouse dendritic cells and other myleoid subtypes in healthy lymph nodes and skin: 26-Color flow cytometry panel for immune phenotyping. Eur J Immunol. 2022 Dec;52(12):2006-2009. doi: 10.1002/eji.202250004. PMID: 35944142
- Bellmann L, Strandt H, Zelle-Rieser C, Ortner D, Tripp CH, Schmid S, Rühl J, Cappellano G, Schaffenrath S, Prokopi A, Spoeck S, Seretis A, Del Frari B, Sigl S, Krapf J, Heufler C, Keler T, Münz C, Romani N, Stoitzner P. Targeted delivery of a vaccine protein to Langerhans cells in the human skin via the C-type lectin receptor Langerin. Eur J Immunol 2022 Nov;52(11):1829-1841. doi: 10.1002/eji.202149670. PMID:
- Leman G, Pavel P, Hermann M, Crumrine D, Elias PM, Minzaghi D, Goudounèche D, Roshardt Prieto NM, Cavinato M, Wanner A, Blunder S, Gruber R, Jansen-Dürr P, Dubrac S. Mitochondrial activity is upregulate in nonlesional atopic dermatitis and amendable to therapeutic intervention. J Invest Dermatol. 2022 Oct;142(10):2623-2634.e12. doi: 10.1016/j.jid.2022.01.035. PMID: 35341734
- André F, Böckle BC. Sjögren-Syndrom. J Dtsch Dermatol Ges. 2022 Jul;20(7):980-1003. doi: 10.1111/ddg.14823_g. PMID:35881105
- Kitchen M, Leierer G, Kistner O, Wodal W, Gisinger M, Zangerle R, Sarcletti M. High seroprotection rates and geometric mean titre increases after repeated annual influenza vaccinations in a cohort of HIV-infected adults in Austria. 2022 Jun 23;40(29):3948-3953. doi: 10.1016/j.vaccine.2022.05.004. PMID: 35606234
- Gruber R, Zschocke A, Zellner H, Schmuth Successful treatment of trichothiodystrophy with Dupilumab. Clin Exp Dermatol. 2021 Oct;46(7):1381-1383. doi: 10.1111/ced.14642. Epub 2021 May 6. PMID: 33955026
- Eckl KM, Gruber R, Brennan L, Marriott A, Plank R, Moosbrugger-Martinz V, Blunder S, Schossig A, Altmüller J, Thiele H, Nürnberg P, Zschocke J, Hennies HC, Schmuth M. Cystatin M/E Variant Causes Autosomal Dominant Keratosis Follicularis Spinulosa Decalvans by Dysregulating Cathepsins L and V. Front Genet. 2021 Jul 12:12:689940. doi: 10.3389/fgene.2021.689940. eCollection 2021. PMID: 34322157
- Pavel P, Leman G, Hermann M, Ploner C, Eichmann TO, Minzaghi D, Radner FPW, Del Frari B, Gruber R, Dubrac S. Peroxisomal fatty acis oxidation and glycolysis are triggerd in mouse models of atopic dermatitis. JID Innov. 2021 Jun 15;1(3):100033. doi: 10.1016/j.xjidi.2021.100033. eCollection 2021 Sep. PMID: 34909730
- Blunder S, Krimbacher T,Moosbrugger-Martinz V, Gruber R, Schmuth M, Dubrac S. Keratinocyte-derived IL1-beta induces PPARG downregulation and PPARD upregulation in human reconstructed epidermis following barrier impairment. Exp Dermatol. 2021 Sep;30(9):1298-1308. doi: 10.1111/exd.14323. Epub 2021 Mar 18.PMID: 33683743.
- Prokopi A, Tripp CH, Tummers B, Hornsteiner F, Spoeck S, Crawford JC, Clements DR, Efremova M, Hutter K, Bellmann L, Cappellano G, Cadilha BL, Kobold S, Boon L, Ortner D, Trajanoski Z, Chen S, de Gruijl T, Idoyaga J, Green DR, Stoitzner P. Skin dendritic cells in melanoma are key for successful checkpoint blockade therapy. J Immunotherap Cancer 2021. Jan;9(1): e000832. PMID:
- Moosbrugger-Martinz V, Hackl H,Gruber R, Pilecky M, Knabl L, Orth-Höller D, Dubrac S. Initial evidence of distinguishable bacterial and fungal dysbiosis in the skin of patients with atopic dermatititis or netherton syndrome. J Invest Dermatol. 2021 Jan;141(1):114-123. doi: 10.1016/j.jid.2020.05.102. Epub 2020 Jun 14.PMID: 32553662.
Selection of Funding
- COST Action CA21108European Network for Skin Engineering and Modelling: NETSKINMODELS (2022-2026) by EU via COST: 800.000€
- FWF-ESPRIT “The role of NLRP3 inflammasome activation in Langerhans Cells for vitiligo” (2022-2025) 316.000 €
- TWF “The role of the NLRP3 inflammasome in Langerhans Cells for skin homeostasis” (2022-2024) 29.000 €
- Graduate Program “Cellular Basis of Diseases” (2021-2024) by Austrian Science Fund (FWF): 200.000 €
- Stand alone Project “Langerhans cell based immunotherapy of cancer with glycomimetic-coated nanoparticles” (2020-2024): P33855 by FWF: 400.000 €
- Stand alone Project “Mitochondria & peroxisomes: novel targets in atopic dermatitis” (2019-2023): P-31662 by FWF: 400.000 €
- Graduate Program „Biomolecular Analyses for Tailored Medicine in AneiNversa (BATMAN)” (2019-2023) by Autrian Science Fund (FWF): Euro 91.230 €
Collaborations
- Hervé Bachelez, INSERM Hopital Saint-Louis, Paris, France
- Michele Boniotto, Institut Mondor for Biomedical Research/INSERM, Créteil, France
- Toni Cathomen, Institute for Transfusion Medicine and Gene Therapy, Medical Center-University of Freiburg, Germany
- Wei-Li Di, Institute of Cell and Molecular Science, Barts and the London School of Medicine and Dentistry, Queen Mary, University of London, UK
- Diana Dudziak, Friedrich-Alexander-University, Erlangen, Germany
- Tanja de Gruijl, Cancer Center of the Amsterdam University, Amsterdam, Netherlands
- Peter Elias, University of California, San Francisco, USA
- Sanja Kezic, UMC Amsterdam, Amsterdam, Netherlands
- Gurjit Khurana Hershey, Cincinnati Children’s Hospital Medical Center, Cincinnati, USA
- Christoph Rademacher, University of Vienna, Austria
- Michel Simon and Corinne Leprince, CNRS-INSERM-University of Toulouse, Toulouse, France
Patent: EP22187107.2: Antisense oligomers and methods for treating atopic dermatitis, psoriasis and other inflammatory conditions- Coinventor (Sandrine Dubrac)