Anichstraße 35
6020 Innsbruck
Fax: +43 (0)50 504 24199
Email: Michael.Joannidis@i-med.ac.at
Website: https://www.i-med.ac.at/notfallmedizin/
Research year
Research Branch (ÖSTAT Classification)
301103, 301203, 301205, 301401, 302030, 302031, 302032, 302053, 301205, 301203
Keywords
Acute kidney injury, antimicrobial agents, biomarkers, coagulation factors, COVID-19, pharmacodynamics, plasma pharmacokinetics, renal replacement therapy, sepsis, and target-site pharmacokinetics
Research Focus
Our work combines applied clinical and translational (bench-to-bedside) research on critical illness, with a particular emphasis on acute kidney injury (AKI), sepsis, and COVID-19. Key areas include:
- Biomarkers – definition and clinical validation for diagnosis and prognosis of AKI and sepsis.
- Renal replacement therapy – optimization of operational strategies in critically ill patients.
- Endothelium–coagulation interactions – identification and characterization of mechanisms driving systemic inflammation and sepsis.
- Pharmacology in critical care – intensive care–specific pharmacokinetics and pharmacodynamics.
General Facts
The Joint Institution / Division of Medical Intensive Care and Emergency Medicine was established in December 2012. Clinically, it was designed as a core facility for the Department of Internal Medicine, to provide a high level of intensive care and emergency medicine. It comprises a level-three intensive care unit and medical emergency room, including a short-stay (maximum of 24 hours) ward in Medizinzentrum Anichstrasse (MZA). Administratively, the Core Facility is affiliated to the Department of Internal Medicine I (Director: Prof. Dr. Herbert Tilg)
The Joint Institution is involved in several multicentre clinical trials to investigate early diagnosis and treatment of acute kidney injury, treatment of severe infections and sepsis, medical emergencies as well as antimicrobial pharmacokinetics. Complementary in vitro models are used to investigate inflammatory mechanisms of renal injury. Basic research is performed at the Joint Institution´s Inflammation Research Laboratory (U-1-015), which hosts three research groups: the Intensive Care Medicine (Mag. Anna Brandtner, PhD, Dr. Paul Köglberger, PD Dr. Georg F Lehner, PhD, Dr. Timo Mayerhöfer, Dr. Fabian Perschinka, Dr. Birgit Zassler, Dr. Andrea Köhler, Sebastian Schauflinger) and Medical Emergency Group (Dr. Frank Hartig, Dr. Nicolas Prokes, Dr. Bernhard Benda, PD Dr. Armin Finkenstedt) led by Univ. Prof. Dr. Michael Joannidis as well as the Clinical Pharmacokinetics (Luisa Guthardt, BSc., Leif Kozik) group led by Ao Univ. Prof. Dr. Romuald Bellmann.
Collaboration partners include all university clinics (I – V) in the Department of Internal Medicine, the Neurological Intensive Care Unit/Department of and the Surgical/Trauma Intensive Care Unit as well as the Hygiene and Medical Microbiology division and the Molecular and Cellular Pharmacology division.
Research
Intensive Care Medicine
Head: Michael Joannidis, MD, Professor
Acute Kidney Injury (AKI)
Timo Mayerhöfer, Fabian Perschinka, Paul Köglberger, Birgit Zassler, Michael Joannidis
Diagnosis and Pathophysiology
AKI affects over 50% of critically ill patients and is linked to high mortality and long-term morbidity, including end-stage renal disease. As AKI plays a central role in organ crosstalk during critical illness, we focus on pathophysiological triggers in patients with respiratory failure. In a matched cohort of more than 8000 patients, we recently demonstrated that invasive mechanical ventilation is an independent risk factor for AKI, regardless of underlying disease (1).
Early diagnosis and reliable prediction of recovery are crucial for improving outcomes. We contributed to several multicentre trials that established novel biomarkers: urinary cell-cycle arrest proteins TIMP-2 and IGFBP-7 for early detection, and chemokine ligand 14 (CCL-14) for predicting persistent AKI. These findings prompted proposals to revise treatment approaches by integrating biomarkers (2).
In complementary studies, we currently investigate how hypoxia and chronic inflammation affect CCL-14 release from renal epithelial cells using an endo-epithelial co-culture system. Such biomarkers may enable individualized therapy by distinguishing reversible from persistent AKI.
Treatment
About 5–10% of AKI patients require renal replacement therapy (RRT). To determine the optimal initiation strategy, we co-designed and coordinated the STARRT-AKI trial, the largest randomized international multicentre study comparing accelerated versus standard RRT initiation. Accelerated treatment increased the risk of long-term dialysis dependence, particularly in patients with chronic kidney disease (3).
Secondary analyses showed that continuous RRT is beneficial for hemodynamically unstable patients (4). Optimal CRRT requires effective anticoagulation; citrate has become the standard due to improved filter lifespan and reduced bleeding risk. Since citrate is metabolized to bicarbonate, it influences acid–base balance: our retrospective study demonstrated rapid correction of acidosis but persistent metabolic alkalosis in patients initially presenting with alkalosis. This effect depends strongly on the bicarbonate content of the substitution fluid (5).
We therefore conducted an investigator-initiated randomized trial (NCT04071171) evaluating substitution fluid composition during citrate-anticoagulated RRT (6). Results are expected to optimize treatment in critically ill patients requiring RRT.
Sepsis
Pathopyhsiology-Thromboinflammation
Georg Lehner, Fabian Perschinka, Anna Tobiasch, Birgit Zassler, Andrea Köhler, Michael Joannidis
Sepsis and septic shock are life-threatening syndromes characterized by a dysregulated host response, organ dysfunction, and mortality rates of up to 43% in septic shock. Key pathophysiological mechanisms involve endothelial injury, inflammation, and coagulation disturbances.
In our lab, we developed an endothelial cell–based coagulation assay mimicking septic inflammation. We found that microvascular endothelial cells exhibit increased tissue factor (TF) activity, not from higher TF expression, but from an imbalance with its inhibitor TFPI. A high TF/TFPI ratio promotes disseminated intravascular coagulation (DIC), microvascular dysfunction, and organ failure.
In a clinical study funded by the Österreichische Gesellschaft für Internistische und Allgemeine Intensivmedizin und Notfallmedizin (ÖGIAIN), we demonstrated that elevated TF/TFPI ratios are linked specifically to myocardial injury markers, whereas high systemic TFPI levels correlate with AKI, liver dysfunction, DIC, and overall disease severity (7). Moreover, extracellular vesicle–associated active TF (rather than total TF antigen) predicted DIC development (Figure 1) (8).
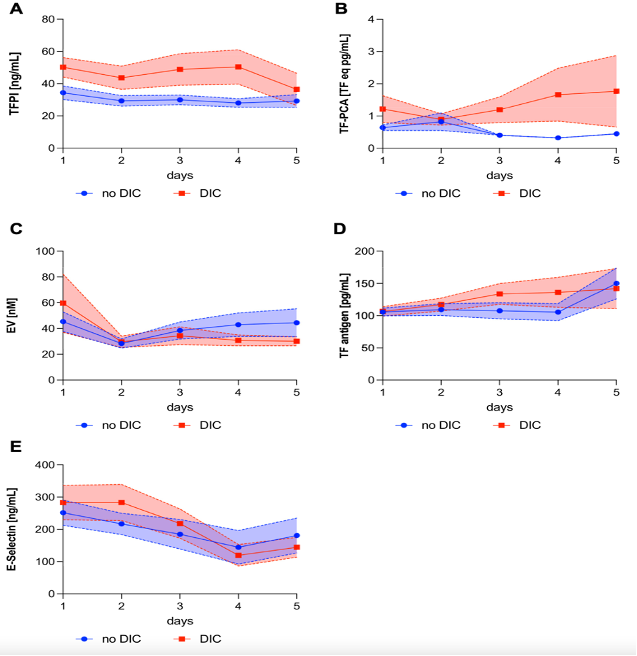
patients who never developed overt disseminated intravascular coagulopathy (DIC) vs patients who reached 5 or more points of the International Society on Thrombosis and Haemostasis DIC score. Lines indicate mean values with the corresponding SE. (Res Pract Thromb Haemost. 2024 Oct 18;8(7):102596. doi: 10.1016/j.rpth.2024.102596.)
Therapeutic plasma exchange (TPE) is a promising approach to modulate coagulation and inflammation. In collaboration with groups from Germany and Switzerland, we showed that TPE removes both TF and TFPI (9). High baseline TF levels predicted treatment response, suggesting that TF may serve as a predictive enrichment biomarker in future trials.
Subphenotyping of Sepsis
Fabian Perschinka, Birgit Zassler, Andrea Köhler, Georg Lehner, Michael Joannidis
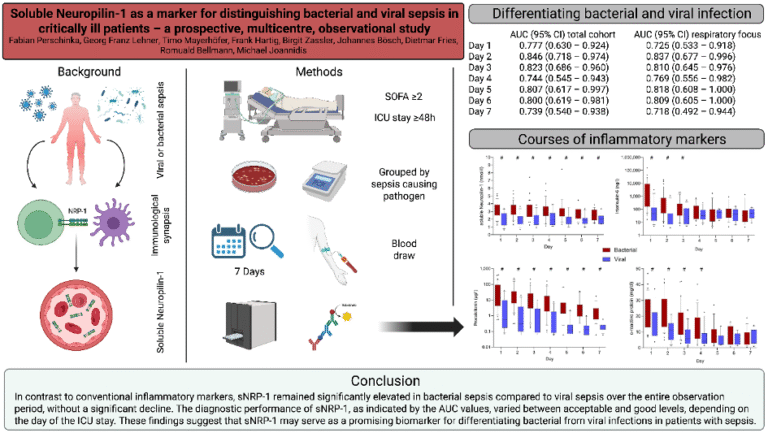
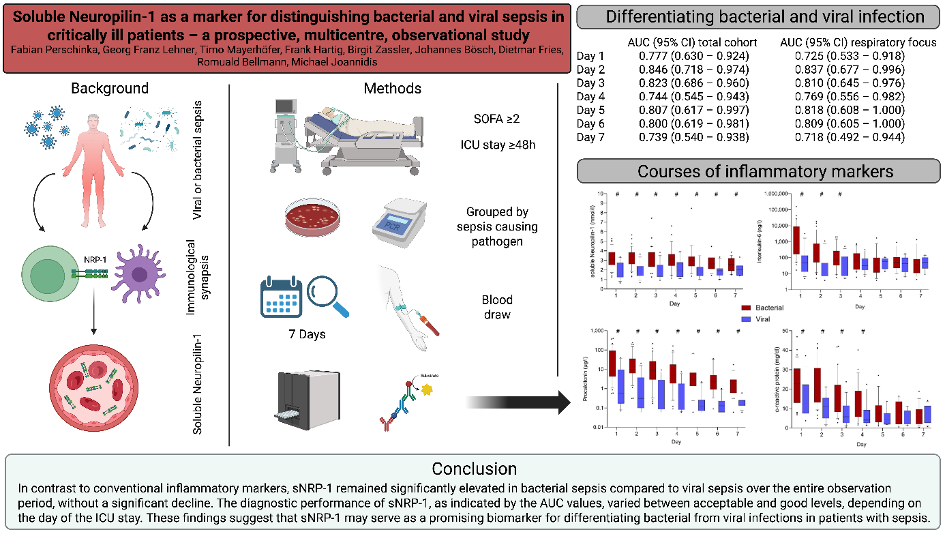
The causative pathogen strongly influences clinical presentation, disease course, and outcomes in sepsis. In COVID-19–associated sepsis, we identified distinct phenotypes compared with bacterial sepsis, with marked differences in IL-6 and procalcitonin levels (10). Extending this analysis to other viral and bacterial sepsis cohorts, we confirmed these findings and identified soluble neuropilin-1 as a novel biomarker discriminating viral from bacterial sepsis (Figure 2) (11). Neuropilin-1, expressed on dendritic cells and regulatory T cells, is pivotal in mediating dendritic–T-cell interactions.
Critical illness in COVID-19
Timo Mayerhöfer, Fabian Perschinka, Georg Lehner, Andreas Peer, Michael Joannidis
SARS-CoV-2 infection carries a high risk of respiratory failure requiring mechanical ventilation. The pandemic surges strained ICU capacity worldwide. With the Tyrol COVID-19 registry established in 2020, we continuously collected clinical data. Analyses showed that a regional ICU network with a tertiary centre as backbone improved outcomes and optimized resource use. Patient transfers within this network proved safe (12).
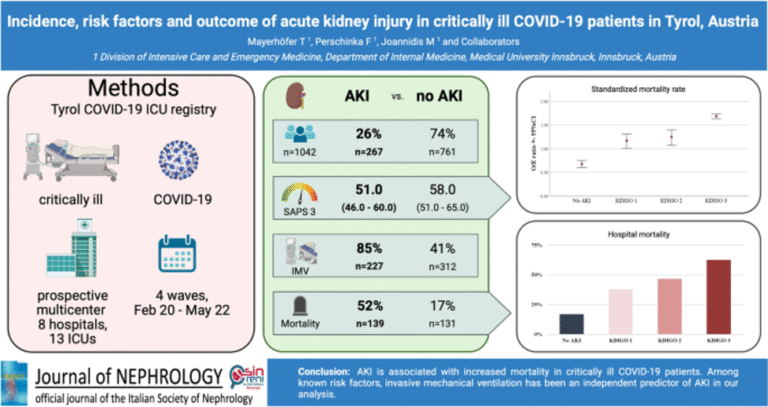
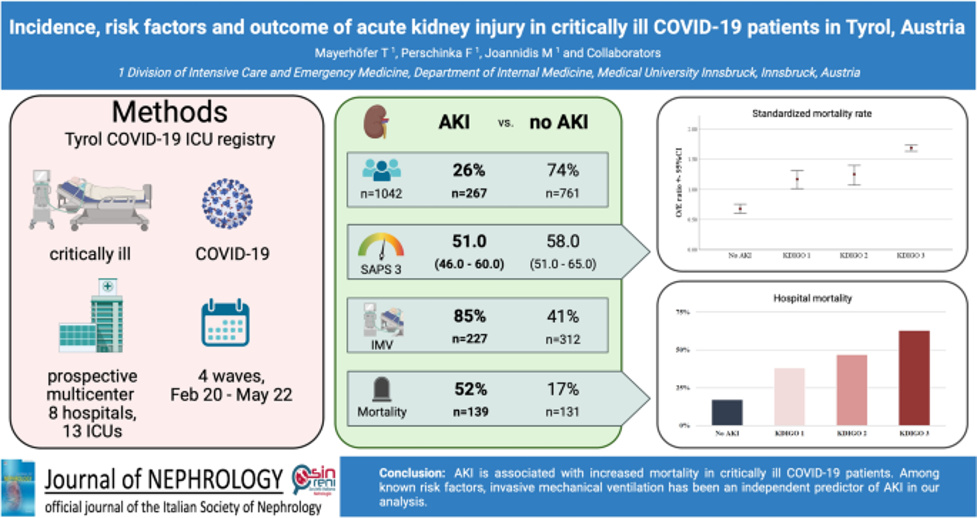
Regarding risk factors, we consistently demonstrated that overt and unrecognized diabetes strongly predispose to severe COVID-19 and poor outcomes, which could be confirmed even a larger specific cohort of elderly multimorbid patients (13). Within the cohort from the Tyrol COVID-19 Registry, we studied AKI incidence and outcomes, showing that AKI was highly prevalent and independently associated with mortality (figure 3) (14), underscoring the importance of early recognition and targeted management.
In patients with COVID-19–associated ARDS (CARDS), venovenous ECMO (vvECMO) was often required(15). Anticoagulation management posed particular challenges. In a bi-centric exploratory study, we showed that argatroban anticoagulation guided by Hemoclot™ was both safe and effective, supporting it as an alternative to heparin (16).
Overall, our studies highlight the complexity of multi-organ involvement in severe COVID-19 and contribute to a deeper understanding of risk factors and therapeutic strategies in critically ill patients.
Emergency Medicine – Emergency Room Management
Frank Hartig, Bernhard Benda, Nicolas Prokes , Armin Finkenstedt, Michael Joannidis
Logistical aspects of emergency care and shock room management are an emerging research focus. We recently established a registry of shock room patients over the past five years.
A critical component of emergency medicine is helicopter transport. By measuring oxygen enrichment in three commonly used helicopter models, we demonstrated significant cabin oxygen accumulation during supplementation in patients with respiratory failure. As elevated oxygen levels increase fire risk, this represents a relevant safety hazard (17).
Pharmacokinetics and Pharmacodynamics of Antifungals in Critical Illness
Luisa Guthardt, BSc., Leif Kozik, Romuald Bellmann
A sound understanding of pharmacokinetics (PK) and pharmacodynamics (PD) is essential for the optimal selection and dosing of drugs in order to ensure efficacy and safety. In critically ill patients, PK is not only influenced by pathophysiological changes but also by drug–drug interactions (18). Invasive fungal infections, particularly invasive mould infections, remain a major threat to immunocompromised patients (19, 20). While several novel antifungals are in clinical and preclinical development, the optimized use of currently available agents remains pivotal for treating these life-threatening conditions (20). We therefore investigate plasma and target-site PK as well as target-site PD of antifungals in critically ill patients.
Echinocandin Target-Site Pharmacokinetics
We determined the PK of the echinocandins anidulafungin, micafungin, and caspofungin in pleural effusion and bile of critically ill patients. Echinocandin concentrations in pleural effusion were consistently lower than in plasma and displayed delayed distribution and elimination (21). Similarly, biliary levels were markedly below simultaneous plasma concentrations (Oberacher et al., in preparation).
Target-Site Pharmacodynamics
To assess echinocandin PD in pleural effusion, we conducted in-vitro experiments with patient-derived pleural effusion samples, spiked with increasing echinocandin concentrations (0–16 mg/L) and inoculated with Candida albicans or C. glabrata. Fungal growth was quantified and compared with that in culture medium. In parallel, ex-vivo experiments were performed by inoculating pleural effusion from study patients with the same strains. Echinocandin concentrations achievable in pleural effusion reduced fungal burden but did not reliably eradicate C. albicans or C. glabrata.
We are currently extending these analyses to bile: Candida albicans and C. glabrata are incubated in echinocandin-spiked porcine bile in-vitro, followed by ex-vivo experiments using bile samples from study patients containing clinically achieved echinocandin concentrations (Guthardt et al., in progress).
Therapeutic Drug Monitoring of Azole Antifungals
The triazoles voriconazole and isavuconazole are recommended for the treatment of invasive aspergillosis, while posaconazole is the drug of choice for antifungal prophylaxis in high-risk patients. Broad-spectrum azoles display complex PK, making therapeutic drug monitoring (TDM) a promising strategy to optimize efficacy and minimize toxicity. At the Medical University of Innsbruck, we are analyzing the clinical use and results of TDM for broad-spectrum azoles. Our studies evaluate whether standard dosing achieves adequate exposure, and whether TDM-guided dose adjustments improve therapeutic target attainment. We further assess the impact of liver function and critical illness on azole PK (Bellmann & Kozik, in progress).
Pictures
Selected Publications
- Perschinka F, Mayerhöfer T, Engelbrecht T, Graf A, Zajic P, Metnitz P, et al. Impact of mechanical ventilation on severe acute kidney injury in critically ill patients with and without COVID-19 – a multicentre propensity matched analysis. Ann Intensive Care. 2025;15(1):17.
- Ostermann M, Forni LG, Joannidis M, Kane-Gill SL, Legrand M, Lumlertgul N, et al. State of the art: Renal recovery after AKI – from basic science to clinical practice. Intensive Care Med. 2025;51(8):1490-507.
- Bagshaw SM, Wald R, Adhikari NKJ, Bellomo R, da Costa BR, Dreyfuss D, et al. Timing of Initiation of Renal-Replacement Therapy in Acute Kidney Injury. N Engl J Med. 2020;383(3):240-51.
- Wald R, Gaudry S, da Costa BR, Adhikari NKJ, Bellomo R, Du B, et al. Initiation of continuous renal replacement therapy versus intermittent hemodialysis in critically ill patients with severe acute kidney injury: a secondary analysis of STARRT-AKI trial. Intensive Care Med. 2023;49(11):1305-16.
- Mayerhöfer T, Köglberger P, Perschinka F, Lehner GF, Schilchegger L, Bellmann R, et al. High bicarbonate replacement fluid and time to pH normalization during continuous veno-venous hemofiltration with regional citrate anticoagulation: a retrospective single-center cohort study. Clin Kidney J. 2025;18(5):sfaf117.
- Köglberger P, Perschinka F, Klein SJ, Mayerhöfer T, Maier S, Ulmer H, et al. Protocol of the comparison of two different bicarbonate replacement fluids during CVVH with RCA: a Prospective, Randomized, Controlled Trial. Blood Purif. 2025:1-20.
- Lehner GF, Tobiasch AK, Perschinka F, Mayerhöfer T, Waditzer M, Haller V, et al. Associations of tissue factor and tissue factor pathway inhibitor with organ dysfunctions in septic shock. Sci Rep. 2024;14(1):14468.
- Tobiasch AK, Lehner GF, Feistritzer C, Peer A, Zassler B, Neumair VM, et al. Extracellular vesicle tissue factor and tissue factor pathway inhibitor are independent discriminators of sepsis-induced coagulopathy. Res Pract Thromb Haemost. 2024;8(7):102596.
- Stahl K, Lehner GF, Wendel-Garcia PD, Seeliger B, Pape T, Schmidt BMW, et al. Effect of therapeutic plasma exchange on tissue factor and tissue factor pathway inhibitor in septic shock. Crit Care. 2024;28(1):351.
- Perschinka F, Mayerhöfer T, Lehner GF, Hasslacher J, Klein SJ, Joannidis M. Immunologic response in bacterial sepsis is different from that in COVID-19 sepsis. Infection. 2022;50(4):1035-7.
- Perschinka F, Lehner GF, Mayerhöfer T, Hartig F, Zassler B, Bösch J, et al. Soluble Neuropilin-1 as a Marker for Distinguishing Bacterial and Viral Sepsis in Critically Ill Patients-A Prospective, Multicenter, Observational Study. Viruses. 2025;17(7).
- Perschinka F, Niedermoser H, Peer A, Lehner GF, Mayerhöfer T, Stöllnberger V, et al. Safety of interhospital transfer for critically ill COVID-19 patients. Crit Care. 2023;27(1):456.
- Mayerhöfer T, Klein S, Wernly B, Flaatten H, Guidet B, De Lange DW, et al. Diabetes mellitus is associated with 90-day mortality in old critically ill COVID-19 patients: a multicenter prospective observational cohort study. Infection. 2023;51(5):1407-15.
- Mayerhöfer T, Perschinka F, Klein SJ, Peer A, Lehner GF, Bellmann R, et al. Incidence, risk factors and outcome of acute kidney injury in critically ill COVID-19 patients in Tyrol, Austria: a prospective multicenter registry study. J Nephrol. 2023;36(9):2531-40.
- Peer A, Perschinka F, Lehner G, Mayerhöfer T, Mair P, Kilo J, et al. Outcome of COVID-19 patients treated with VV-ECMO in Tyrol during the pandemic. Wien Klin Wochenschr. 2024;136(15-16):465-71.
- Mayerhöfer T, Joannidis M, Peer A, Perschinka F, Fries D, Mair P, et al. Anticoagulation with argatroban using hemoclot™ targets is safe and effective in CARDS patients receiving venovenous extracorporeal membrane oxygenation: An exploratory bi-centric cohort study. Thromb Res. 2024;236:161-6.
- Kohler LM, Köhler A, Perschinka F, Benda BM, Joannidis M, Hartig F. Oxygen accumulation and associated dangers in rescue helicopters. BMC Emerg Med. 2024;24(1):146.
- Bellmann R, Weiler S. [Drug-drug interactions in critically ill patients]. Med Klin Intensivmed Notfmed. 2024.
- Egger M, Bellmann R, Krause R, Boyer J, Jakšić D, Hoenigl M. Salvage Treatment for Invasive Aspergillosis and Mucormycosis: Challenges, Recommendations and Future Considerations. Infect Drug Resist. 2023;16:2167-78.
- Hurraß J, Heinzow B, Walser-Reichenbach S, Aurbach U, Becker S, Bellmann R, et al. [Medical clinical diagnostics for indoor mould exposure – Update 2023 (AWMF Register No. 161/001)]. Pneumologie. 2024;78(10):693-784.
- Marx J, Reinstadler V, Gasperetti T, Welte R, Oberacher H, Moser P, et al. Human tissue distribution of caspofungin. Int J Antimicrob Agents. 2022;59(4):106553.
Selection of Funding
- COVID-19 ICU Tyrol Registry funded by the Tiroler Landesregierung (Project F.45824, Michael Joannidis)
- Tissue factor and tissue factor pathway inhibitor in sepsis – ÖGIAIN Research fund 2023 (Georg Lehner)
- Pathophysiology of Lung Kidney injury – ÖGIAIN Research fund 2025 (Timo Mayerhöfer)
- Target-Site Pharmacokinetics and –Activity of Echinocandins, Austrian Research funds FWF (Project KLI 565-B31), Romuald Bellmann
- The study on pharmacokinetics of trimethoprim and sulfametrole was supported Austrian Research Promotion Agency (FFG, project number 848350) and by an industrial grant.
Collaborations
- Univ. Prof. Dr. Thomas Staudinger, Intensive Care Unit, Internal Medicine I, Medical University Vienna, Vienna, Austria
- PD. Dr. Philipp Eller, Department of Internal Medicine, University Hospital of Graz
- Martin Hoenigl, Division of Infectious Diseases, Excellence Center for Medical Mycology (ECMM), Medical University of Graz, Graz, Austria, Division of Infectious Diseases and Global Public Health, Department of Medicine, University of California San Diego, La Jolla, CA, USA and Working Group, University of California San DiegoClinical and Translational Fungal , La Jolla, CA, USA.
- Dr. Daniel Dankl, Salzburg General Hospital and Paracelsus Private Medical University, Salzburg
- Dr. Piotr Smuszkiewicz, Department of Anesthesiology, Intensive Therapy and Pain Treatment, University Hospital Przybyszewskiego, Poznan, Poland
- PD. Dr. Stefan Weiler, Pharmacoepidemiology unit, Institute for Pharmaceutical Sciences, ETH Zurich, Zurich, Switzerland.
- Dr. Julia Hurraß, Section for Hygiene in Healthcare Facilities, Division of Infection Control and Environmental Hygiene, Cologne Health Department, Cologne
- Prof. Stefan Kluge, Department of Intensive Care Medicine, Hamburg Eppendorf, Hamburg, Germany
- Prof Lui Forni, 2 School of Medicine, University of Surrey, Guildford, Surrey, UK
- John Kellum, MD, FCCM, FACP, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA
- Sean Bagshaw MD, PhD , Assoc. Prof., University of Alberta, Edmonton, Canada
- Peter Pickkers, Department of Intensive Care, Radboud University Medical Centre, Nijmegen, the Netherlands
- Ravindra L. Mehta, MD, UCSD Medical Centre, San Diego, CA, USA
- Niklas Nielsen, MD. Lund University, Sweden