Anichstraße 35
6020 Innsbruck
Fax: +43 (0)50 504 23538
Email: Herbert.Tilg@i-med.ac.at
Website: https://inneremed1.tirol-kliniken.at/ _
Research year
Research Branch (ÖSTAT Classification)
301904, 302012, 302014, 302016, 302025, 302030
Keywords
endocrinology, Gastroenterology, hepatology, Inflammation, inflammatory bowel disease, metabolic dysfunction-associated steatotic liver disease, metabolism, microbiota, and nutrition
Research Focus
The Department is dedicated to translational research in the fields of gastroenterology, hepatology, endocrinology and metabolism with a particular emphasis on inflammatory mechanisms. Our scientific activities are driven by a clear objective: to gain deeper insights into the pathophysiology of prevalent diseases. These include inflammatory bowel disease, metabolic and genetic liver diseases, obesity, type 2 diabetes and atherosclerosis. Better knowledge will clearly help to improve clinical management of these patients.
General Facts
The Department of Internal Medicine I focuses on four key medical areas: gastroenterology, hepatology, endocrinology and metabolism. Our division has approximately 59 employees and many members, including students, are involved in clinical work and research. Our laboratories are state of the art, and our researchers are able to conduct cutting-edge research in the field of cellular and molecular work. Our research activities are focused on two key aims: to improve knowledge in the respective disease areas and to improve patient care.
Our department hosts one Christian Doppler research laboratory and one laboratory funded by an ERC Starting Grant:
- ERC STG laboratory on mucosal immunology (Univ.-Prof. Dr. Timon E. Adolph)
- Christian Doppler research laboratory on iron and phosphate biology (Univ.-Prof. Dr. Heinz Zoller)
Our research has been funded by the Austrian Research Promotion Agency (FFG), Austrian Science Fund (FWF), Christian Doppler Research Association (CDG) and European Union (FP7) for a number of years. It has been published in highly respected international journals, including Nature Reviews Immunology, Nature Communications, New England Journal of Medicine, Cell Host Microbe, Gastroenterology, Gut, JAMA and many others.
Research
Microbiota and Gastrointestinal Disorders
Maria Effenberger/Herbert Tilg
Our research group has been at the forefront of microbiome research for over a decade. A major microbiome project has been published recently (Si Y et al. Nat Aging 2022). In this study, we demonstrated that long-term life history affects the gut microbiota. This provides clear insights into how lifestyle variables influence and maintain a healthy gut microbiota in later life. Another major interest is the role of the gut microbiome in chronic liver diseases and hepatocellular carcinoma (Effenberger M et al. Hepatol Commun 2023; 7:e00182).
The following microbiome projects are currently in progress:
- particularly metabolome in chronic liver diseases and hepatocellular carcinoma (in collaboration with K. Aden, Kiel, Germany)
- CHRIS study: The study conducted in collaboration with EURAC and V. Temaroli (Gothenburg, Sweden) and Nicola Segata, (Trento, Italy) proves the pathophysiological relevance of gut microbiome and metabolites in type 2 diabetes and MASLD.
In recent years, our research group has published several highly ranked papers. Herbert Tilg is a highly cited researcher (HCR) 2020-2024 (H-Index Web of Science: 108).
Endocrinology and Metabolism
Susanne Kaser
Our group is dedicated to translational research in the fields of obesity, insulin resistance and type 2 diabetes and endocrine disorders.
Diabetes mellitus is a leading cause of death worldwide, affecting more than 800,000 people in Austria. It is estimated that a further 300,000 people in Austria are suffering from prediabetes. This places them at a very high risk of being diagnosed with diabetes within the next few years. There are several well-known risk factors for type 2 diabetes. These include strong genetic predisposition, overnutrition or unhealthy diet, a sedentary lifestyle and smoking. Type 2 diabetes accounts for more than 90% of all diabetes cases.
Our recent study demonstrated that SGLT-2 inhibitors effectively prevent fatty liver disease, insulin resistance and excess weight gain in high-risk individuals (Radlinger B et al, Diabetologia, 2023; 66:754). Empagliflozin treatment has been found to have beneficial effects on adipose tissue function and hepatic insulin signalling. It also positively affects the size and morphology of mitochondria in skeletal muscle. We are currently researching the effects of SGLT-2 inhibitors on the crosstalk between metabolically relevant organs and tissues. Furthermore, we are studying the regulation of glucose transporters in various tissues to better understand their role in metabolic syndrome. Our ongoing studies provide answers to the following questions: what are the metabolic consequences of various commonly used diets in relevant tissues, and how do sex differences in hepatic energy metabolism affect us?
Clinical research in the field of diabetology is currently focused on the clinical and metabolic characterisation of patients with type 2 diabetes and prediabetes. The AUSTRO-PROFIT study, an observational trial conducted in cooperation with the Medical University of Graz, established that metabolic targets were achieved in only a small proportion of patients with type 2 diabetes (Sourij H et al, Kaser S, DOM, 2025). There were remarkably marked gender differences in the disfavour of females. Ongoing studies will improve estimations of undiagnosed diabetes and prediabetes prevalence.
In the field of endocrinology, we are particularly interested in hormonal dysfunction in pituitary disorders. In ongoing studies, we define the hormonal consequences of radiographic findings of empty sella. We also performed other clinical studies in the fields of metabolism and endocrinology in cooperation with the Departments of Neurology, Vascular Surgery and Paediatrics.
Intestinal Inflammation
Timon Adolph
The Department of Internal Medicine I and Gastroenterology laboratory is funded by the ERC. It is our goal to resolve the impact of diet on inflammatory diseases of the gastrointestinal tract. We are particularly focused on understanding the pathophysiology of inflammatory bowel disease.
In a stand-alone project funded by the FWF in 2020, we identified the role of intestinal epithelial glutathione peroxidase 4 (GPX4) in intestinal homeostasis. We discovered that a three-months Western diet causes a Crohn’s disease-like phenotype in GPX4-deficient mice. In our work, published in Nature Communications (Mayr L. et al., Nature Communications 2020, 11, 1775), we report that GPX4 restricts a cytokine response – specifically interleukin 6 (IL-6) and C-X-C Motif Chemokine Ligand 1 (CXCL1) expression – from intestinal epithelial cells (IECs), which is elicited by dietary polyunsaturated fatty acids (PUFAs).We have identified that dietary PUFAs (contained in meat, eggs and oils) can trigger a phenotype similar to Crohn’s disease in mice with reduced epithelial GPX4 activity. We have also identified a mechanism that triggers this dietary polyunsaturated fatty acid-induced phenotype in the intestinal epithelium. This was published in Gastroenterology 2022 (Schwärzler J and Mayr L et al Gastroenterology 162(6):1690-1704).We recently proposed the concept of diet-induced metabolic gut inflammation in Nat Rev Gastroenterol & Hepatol. (Adolph TE et al. Nat Rev Gastroenterol Hepatol. 2022 19(12):753-767).
Current studies delineate mechanistic aspects of autophagy (covered by an FWF stand-alone project until 2024) and other cellular hubs (funded by the ERC until 2027 and an FWF “Forschungsgruppen (FG)” projectGrant) that controls dietary lipid-induced gut inflammation and its relevance in IBD. The funding allows the employment of one post-doc, three PhD students and several MD students (marginal employment) over the next few years. Timon Adolph’s election to a member of the Österreichische Akademie der Wissenschaften (Young ÖAW, 2022) and a Rising Star award from the European Society of Gastroenterology (2023) cemented his scientific excellence. Our science is also brought to public attention in Austrian and German news magazines (“Die Presse”, “ORF”, “Der Standard”, “Tiroler Tageszeitung”, “Österreichische Ärztezeitung”, “Der Spiegel”, “Radio Berlin Brandenburg” and the TV service “Bayrischer Rundfunk”).
The Gastroenterology laboratory’s young researchers have been awarded significant research prizes and secured further funding as a result of their outstanding achievements. Dr. Felix Grabherr received the Wissenschaftspreis des Landes Vorarlberg and a project funded by the European Crohn’s and Colitis Organisation,. Dr. Julian Schwärzler received the Liechtensteinpreis (MUI), a Zukunftspreis from the German Society of IBD and a “Wissenschaftspreis” from the Austrian Society of Gastroenterology & Hepatology.
Hepatology
Heinz Zoller
Research in the Hepatology group focuses on three main aspects, namely iron-related disorders, genetic liver diseases and liver transplantation outcomes.
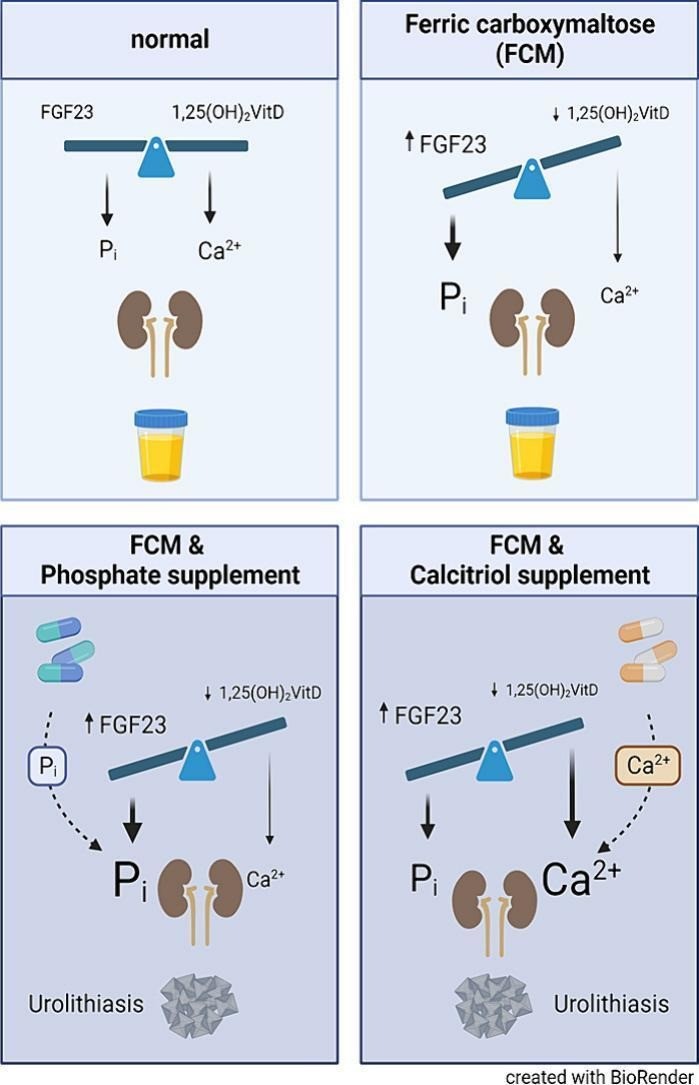
The Hepatology Laboratory in Innsbruck is dedicated to the research of iron metabolism and its effects on liver health. Our key focus is the study of genetically determined liver diseases and the interaction between iron and phosphate metabolism. Another emerging field of expertise is iron deficiency and its consequences on health. In 2019, the Christian Doppler Laboratory for Iron and Phosphate Biology was established at the Medical University of Innsbruck. This laboratory investigates the relationship between iron deficiency, intravenous iron treatment, and the potential reduction of phosphate levels as a result. One of its main research objectives is to identify potential new drugs for bone health. Research in our lab has also described kidney stones as a novel clinical manifestation of hypophosphatemia induced by ferric carboxymaltosis. This work was awarded (Panzer M, et al. Intravenous iron-induced hypophosphatemia and kidney stone disease. Bone Rep. 2024 Mar 29;21:101759. doi: 10.1016/j.bonr.2024.101759.).
The Hepatology Laboratory in Innsbruck has made major contributions to our understanding of genetic liver diseases. We focus on the genetic factors that predispose individuals to conditions such as iron overload disorders and end-stage liver disease. Their research has been instrumental in identifying genetic markers associated with these conditions, enhancing both diagnosis and treatment strategies. The laboratory’s involvement in the development of the European Association for the Study of the Liver’s clinical practice guidelines on haemochromatosis is a significant achievement. This common genetic disorder leads to excessive iron accumulation. This work has provided standardised approaches for the diagnosis and management of haemochromatosis across Europe. A study on the Epidemiology of Haemochromatosis in Western Austria has further elucidated the clinical and biochemical penetrance of haemochromatosis. (Schaefer B, et al. Penetrance, cancer incidence and survival in HFE haemochromatosis-A population-based cohort study. Liver Int. 2024 Mar;44(3):838-847. doi: 10.1111/liv.15797.).
The team has also explored the systemic impact of liver diseases in collaboration with a European research collaboration, particularly focusing on alpha-1-antitrypsin deficiency, a genetic condition affecting both the liver and lungs. Their research has contributed to our understanding of how liver-secreted proteins influence the function of extrahepatic organs (Fromme M, et al. Longitudinal Evaluation of Individuals With Severe Alpha-1 Antitrypsin Deficiency (Pi∗ZZ Genotype). Gastroenterology. 2025 Feb;168(2):367-381. doi: 10.1053/j.gastro.2024.10.010.).
Neurological Implications: In collaboration with other researchers, the team investigated neurodegeneration in hepatic and neurological manifestations of Wilson disease. The study utilised advanced imaging techniques to assess subcortical brain regions, providing clear insights into the neurological impact of the disease (Viveiros A, et al. Neurodegeneration in Hepatic and Neurologic Wilson’s Disease. Hepatology. 2021 Aug;74(2):1117-1120. doi: 10.1002/hep.31681.).
Genetic Diagnosis Challenges: The laboratory explored the limitations of relying solely on genetic testing for diagnosing Wilson disease. Their findings indicate that even individuals carrying two disease-causing mutations might not exhibit altered copper metabolism, emphasising the need for comprehensive clinical evaluation alongside genetic screening (Panzer M, Viveiros A, et al. Synonymous mutation in adenosine triphosphatase copper-transporting beta causes enhanced exon skipping in Wilson disease. Hepatol Commun. 2022 Jul;6(7):1611-1619. doi: 10.1002/hep4.1922.).
The research into aceruloplasminemia is focused on iron chelation therapy: The team studied the efficacy of the oral iron chelator deferasirox in treating aceruloplasminemia, a rare genetic disorder characterised by iron accumulation in the liver and brain due to ceruloplasmin deficiency. The research clearly demonstrated that while deferasirox effectively reduced hepatic iron levels, it did not significantly impact brain iron concentrations. This highlights the challenges in managing neurological aspects of the disease.
Genetic Variants and Iron Overload: The laboratory investigated the role of ceruloplasmin gene variants in iron metabolism disorders. Their research suggested that certain ceruloplasmin mutations may contribute to hyperferritinemia and iron overload in conditions like non-alcoholic fatty liver disease (NAFLD) and haemochromatosis, indicating a complex interplay between genetic factors in iron homeostasis (Zoller H, Tilg H. Ferritin-a promising biomarker in MASLD. Gut. 2024 Apr 5;73(5):720-721. doi: 10.1136/gutjnl-2023-331848.).
The Hepatology Laboratory in Innsbruck has enhanced the understanding of Wilson disease and aceruloplasminemia through these studies, contributing to improved diagnostic approaches and treatment strategies for these complex genetic disorders.
Atherosclerosis
Andreas Ritsch
Our research focused on the role of reverse cholesterol transport in atherosclerosis. Recently, we investigated structure-function-disease relationships of HDL in healthy subjects and in patients with diabetes (T2DM) or coronary heart disease (CHD). We have demonstrated that the various functions of HDL in cells are only loosely linked and are governed by different structural components (Cardner M, et al., JCI Insight. 2020 Jan 16; 5(1): e131491). In pharmacological studies of ApoE knockout rabbits, an animal model for atherosclerosis, we showed that Matcha green tea enhances atherosclerosis in these animals, due to impaired reverse cholesterol transport (Monika Hunjadi, et al. Mol Nutr Food Res 2021 Oct;65(20):e2100371). Our findings from the large-scale clinical study (Young Finns study) demonstrated an inverse correlation between HDL cholesterol efflux capacity and subclinical cardiovascular risk markers in young adults (Hunjadi M, et al., Scientific Reports 5 November 2020 ; 10(1): 19223.). The LURIC study, which involved 2,468 participants, demonstrated that cholesterol efflux is associated with HDL composition as well as an inflammatory burden in patients referred for coronary angiography. This association serves as an inverse predictor of cardiovascular mortality, independent of HDL cholesterol (Ritsch A, et al., Biomedicines. 2020 Nov 21; 8(11): 524). Our recent studies have identified and characterised new players in reverse cholesterol transport. These studies focused on transporters ABCA6 and ABCA8. We successfully established transgenic endothelic, monocytic and hepatic cell lines with enhanced or depressed expression of these reporters. We characterise these cell lines using RNA profiling, measuring HDL-mediated cholesterol efflux and monocytic cell adhesion to endothelial cells, addressing a very early phase in the development of atherosclerosis.
Pictures
Selected Publications
- Effenberger M, Widjaja AA, Grabherr F, Schaefer B, Grander C, Mayr L, et al. Interleukin-11 drives human and mouse alcohol-related liver disease. Gut. 2023;72(1):168-79.
- Effenberger M, Waschina S, Bronowski C, Sturm G, Tassiello O, Sommer F, et al. A gut bacterial signature in blood and liver tissue characterizes cirrhosis and hepatocellular carcinoma. Hepatol Commun. 2023;7(7).
- Grander C, Meyer M, Steinacher D, Claudel T, Hausmann B, Pjevac P, et al. 24-Norursodeoxycholic acid ameliorates experimental alcohol-related liver disease and activates hepatic PPARgamma. JHEP Rep. 2023;5(11):100872.
- Tilg H, Byrne CD, Targher G. NASH drug treatment development: challenges and lessons. Lancet Gastroenterol Hepatol. 2023;8(10):943-54.
- Tilg H, Ianiro G, Gasbarrini A, Adolph TE. Adipokines: masterminds of metabolic inflammation. Nat Rev Immunol. 2024.
- Zollner A, Koch R, Jukic A, et al. Clearance of gut mucosal Sars-Cov2-antigens and post-acute Covid-19 after 2 years in patients with inflammatory bowel disease. Gastroenterology 2024; 167:604-607.
- Radlinger B, Ress C, Kaser S, Tilg H et al. Empagliflozin protects mice against diet-induced obesity, insulin resistance and hepatic steatosis. Diabetologica. 2023; 66:754-767.
- Adolph TE, Tilg H. Western diets and chronic diseases. Nat Med. 2024 Aug;30(8):2133-2147. doi: 10.1038/s41591-024-03165-6. Epub 2024 Jul 31. PMID: 39085420.
- Adolph TE, Meyer M, Jukic A, Tilg H. Heavy arch: from inflammatory bowel diseases to metabolic disorders. Gut. 2024 Jul 11;73(8):1376-1387. doi: 10.1136/gutjnl-2024-331914. PMID: 38777571; PMCID: PMC11287632.
- Schwärzler J, Mayr L, Grabherr F, Tilg H, Adolph TE. Epithelial metabolism as a rheostat for intestinal inflammation and malignancy. Trends Cell Biol. 2024 Nov;34(11):913-927. doi: 10.1016/j.tcb.2024.01.004. Epub 2024 Feb 10. PMID: 38341347.
- Dai E, Chen X, Linkermann A, Jiang X, Kang R, Kagan VE, Bayir H, Yang WS, Garcia-Saez AJ, Ioannou MS, Janowitz T, Ran Q, Gu W, Gan B, Krysko DV, Zhu X, Wang J, Krautwald S, Toyokuni S, Xie Y, Greten FR, Yi Q, Schick J, Liu J, Gabrilovich DI, Liu J, Zeh HJ, Zhang DD, Yang M, Iovanna J, Kopf M, Adolph TE, Chi JT, Li C, Ichijo H, Karin M, Sankaran VG, Zou W, Galluzzi L, Bush AI, Li B, Melino G, Baehrecke EH, Lotze MT, Klionsky DJ, Stockwell BR, Kroemer G, Tang D. A guideline on the molecular ecosystem regulating ferroptosis. Nat Cell Biol. 2024 Sep;26(9):1447-1457. doi: 10.1038/s41556-024-01360-8. Epub 2024 Feb 29. PMID: 38424270; PMCID: PMC11650678.
- Drakesmith H, Zoller H. The iron curve: infection at both ends. Blood. 2024 Aug 15;144(7):679-680. doi: 10.1182/blood.2024025259. PMID: 39145941.
- Sanyal AJ, Bedossa P, Fraessdorf M, Neff GW, Lawitz E, Bugianesi E, Anstee QM, Hussain SA, Newsome PN, Ratziu V, Hosseini-Tabatabaei A, Schattenberg JM, Noureddin M, Alkhouri N, Younes R; 1404-0043 Trial Investigators. A Phase 2 Randomized Trial of Survodutide in MASH and Fibrosis. N Engl J Med. 2024 Jul 25;391(4):311-319. doi: 10.1056/NEJMoa2401755.
- Panzer M, Meindl E, Schaefer B, Wagner S, Glodny B, Mayer G, Pircher A, Schwarz C, Beckmann F, Hejny C, Joachim-Mrosko B, Konzett J, Tilg H, Heidegger I, Wolf M, Weiskirchen R, Zoller H. Intravenous iron-induced hypophosphatemia and kidney stone disease. Bone Rep. 2024 Mar 29;21:101759. doi: 10.1016/j.bonr.2024.101759. PMID: 38590391; PMCID: PMC10999795.
- Zoller H, Tilg H. Ferritin-a promising biomarker in MASLD. Gut. 2024 Apr 5;73(5):720-721. doi: 10.1136/gutjnl-2023-331848. PMID: 38538068.
- Fromme M, Hamesch K, Schneider CV, Mandorfer M, Pons M, Thorhauge KH, Pereira V, Sperl J, Frankova S, Reichert MC, Benini F, Burbaum B, Kleinjans M, Amzou S, Rademacher L, Bewersdorf L, Verbeek J, Nevens F, Genesca J, Miravitlles M, Nuñez A, Schaefer B, Zoller H, Janciauskiene S, Waern J, Oliveira A, Maia L, Simões C, Mahadeva R, Fraughen DD, Trauner M, Krag A, Lammert F, Bals R, Gaisa NT, Aigner E, Griffiths WJ, Denk H, Teumer A, McElvaney NG, Turner AM, Trautwein C, Strnad P. Alpha-1 Antitrypsin Augmentation and the Liver Phenotype of Adults With Alpha-1 Antitrypsin Deficiency (Genotype Pi∗ZZ). Clin Gastroenterol Hepatol. 2024 Feb;22(2):283-294.e5. doi: 10.1016/j.cgh.2023.08.038. Epub 2023 Sep 15. PMID: 37716616.
- Maria Noflatscher, Monika Hunjadi, Michael Schreinlechne , Philip Sommer, Daniela Lener, Markus Theurl, Rudolf Kirchmai, Axel Bauer, Andreas Ritsch, Peter Marschang. Inverse Correlation of Cholesterol Efflux Capacity with Peripheral Plaque Volume Measured by 3D Ultrasound. Biomedicines. 2023 Jul 6;11(7):1918. doi: 10.3390/biomedicines11071918
Patents
IL-11 and alcoholic liver disease (Effenberger, Tilg)
Gpx4 and postoperative Crohn´s disease (Adolph, Schwärzler, Tilg
Selection of Funding
- Funding is also supported by the excellence initiative VASCage (Centre for Promoting Vascular Health in the Ageing Community), an R&D K-Centre (COMET program – Competence Centers for Excellent Technologies) funded by the Austrian Ministry for Transport, Innovation and Technology, the Austrian Ministry for Digital and Economic Affairs and the federal states Tyrol, Salzburg and Vienna. (Herbert Tilg)
- Christian-Doppler-Research Laboratory for Iron and Phosphate Biology (Univ.-Prof. Dr. Zoller)
- FWF Forschungsgruppe FG15 2022-2026 (Timon Adolph)
- ERC Starting Grant 2022-2026 (Timon Adolph)
- MYCOS PhD consortium 2024-2027 (Timon Adolph)
- AI-Grant from Tyrolean Government to Benedikt Schaefer and Maria Effenberger
- TWF Grant – Marlene Panzer
Collaborations
- Arthur Kaser, Cambridge
- PD Dr. Konrad Aden, Kiel
- Richard Steven Blumberg, Boston Harvard Medical School, Gastroenterology, Hepatology & Endoscopy
- Michael Roden, Düsseldorf, Endocrinology
- Erwin Wagner, PhD, Medical University Vienna
- Michael Trauner, Medical University Vienna
- Percy A. Knolle, TUM Technical University Munich
- Myles S Wolf, Columbia University, New York, USA
- Ralf Weiskirchen, Rheinisch Westfählische Technische Hochschule, Aachen, Germany
- Markus Hartmann, Ludwig Boltzman Institut für Osteologie, Vienna, Austria
- Pavel Strnad, University Hospital of Aachen, Germany