Innrain 80
6020, Innsbruck
Email: hesso.farhan@i-med.ac.at
Website: http://www.pathophysiology-innsbruck.com/
Research year
Research Branch (ÖSTAT Classification)
106052, (33.33%) 301109, (33.33%) 301904 (33.33%)
Keywords
Aneuploidy, Breast cancer, Cancer metastasis., Endoplasmic Reticulum, Mechanobiology, membrane trafficking, Mitosis, Multiple myeloma, Proteostasis, and Ubiquitin ligases
Research Focus
The group’s main research focus is on how mechanobiology regulates cellular membranes, such as the endoplasmic reticulum (ER) and plasma membrane-derived extracellular membranous tracks. We investigate a range of diseases models, including breast cancer and multiple myeloma. Additional research lines focus on the regulation of mitosis, cell migration and on the crosstalk of pseudoenzymes with F-box proteins.
General Facts
Two research groups work at the Institute of Pathophysiology:
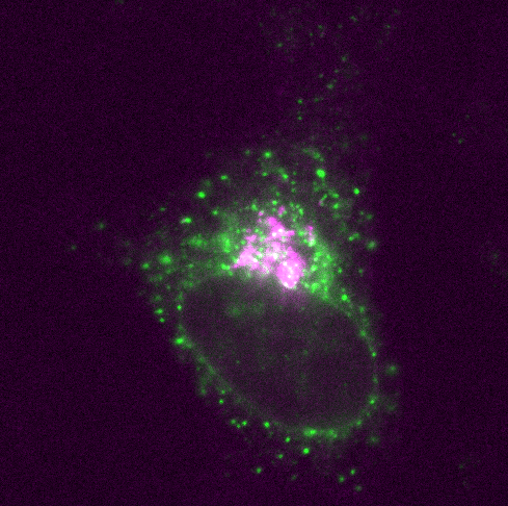
- The Farhan group is dedicated to the connection between mechanobiology and membrane trafficking, along with ER-proteostasis (Figure 2)
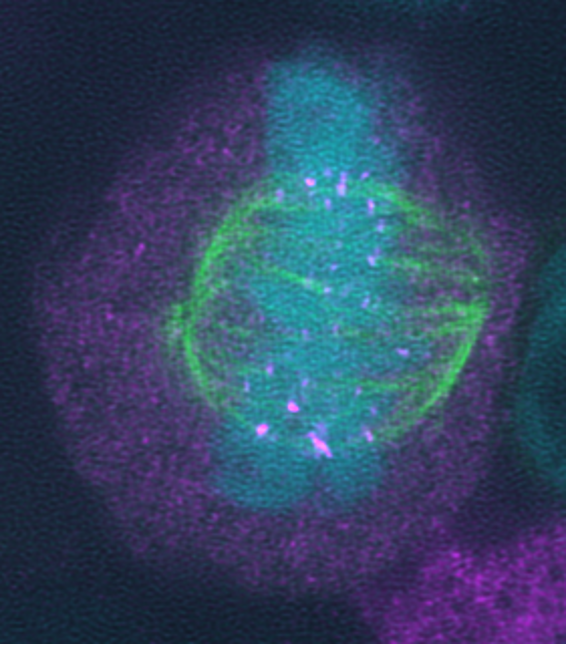
The focus is also on the pseudophosphatase STYX and its exchange with F-box proteins and how they regulate the spindle assembly checkpoint (Figure 3).
- The Baschieri group investigates the roles of mechanobiology and endocytosis in cancer cell invasion, migration and metastasis.
Research
Below is a list of the range of research topics at the Institute of Pathophysiology and the people who perform experiments in support of this topic:
-
Regulation of Endoplasmic reticulum (ER) proteostasis
One-third of the proteome is composed of secretory proteins. The endoplasmic reticulum (ER) is the first checkpoint on the secretory journey of a protein, thus making the ER a major hub for protein homeostasis (proteostasis). Our research focuses squarely on the mechanisms that connect cell growth and quiescence with the regulation of ER morphology and function. We are also investigating lectin-type cargo receptors such as ERGIC-53 (LMAN1), VIP36 (LMAN2) and VIPL (LMAN2L). It is our aim to identify novel cargos that use these receptors for the exit from the ER. Finally, we are committed to unravelling the role of ER-export in diseases such as multiple myeloma. This type of cancer is characterised by excessive secretory activity. It is our goal to pinpoint the critical components of the ER-export machinery that are essential to myeloma cells and thereby identify novel and effective therapeutic targets.
The following members of Pathophysiology are involved: Utku Horzum, Yannick Frey, Veronika Reiterer, Laura Sammarco, Margot Haun, Gabriele Stöckl
-
Pseudophosphatases as regulators of F-box Proteins:
All major enzyme families contain members with mutations in conserved domains that predict alterations to their catalytic activity. These members are known as pseudoenzymes. In the human phosphatome, pseudophosphatases make up 14% of all phosphatases. We are investigating STYX, an archetype pseudophosphatase. Our working hypothesis is that the loss of enzymatic activity allows pseudophosphatases like STYX to adopt new cellular functions that go beyond crosstalk with kinases. We discovered that STYX regulates several F-box proteins (FBPs). FBPs are part of ubiquitin-ligase complexes. Their function is to bind the substrates destined for ubiquitylation. We are characterising the role of STYX in the regulation of several F-box proteins, including FBXW7, FBXO31, FBXL12 and FBXW4.
The following members of Pathophysiology are involved: Veronika Reiterer-Farhan, Margot Haun
-
ER-Proteostasis in health and diseases:
Our cells are constantly exposed to a variety of mechanical forces. These include tensile stress, hydrostatic pressure, compression and confinement. Mechanosensors sense these forces and convert them into biochemical signals that regulate cellular functions (mechanotransduction). The community’s main focus was on the role of the plasma membrane and the nucleus in mechanosensing and mechanotransduction. Our previous work (Phuyal et al, EMBOJ, 2022) showed that mechanical forces elicit signalling pathways that regulate export from the ER. Our current work tests the hypothesis that the ER can sense mechanical forces and elicit mechanotransduction signalling from the ER. The ER is the largest cellular organelle, spanning almost the entire cellular area. It is therefore conceivable that most mechanical forces will also affect the structure and function of the ER. We are currently identifying and characterising an ER-based mechanosensory, as well as the lipidomic changes of the ER under mechanical stress.
The following members of Pathophysiology are involved: Michaela Mayr, Alexander Plesche, Luiz Garcia
-
Tumour microenvironment and cancer cell adhesion:
Changes in the microenvironment are critical in cancer progression. Locally activated fibroblasts, known as cancer-associated fibroblasts (CAFs), are key to altering the extracellular matrix’s protein composition, rearranging its structure, and influencing various physical characteristics of the tissue. We have recently demonstrated that CAFs leave tracks among their migration paths, specialised extracellular vesicles that enhance cancer cell adhesion through a specific type of adhesive structure called endocytosis-related adhesions. Cancer cells adhere to and internalise these tracks, along with their content. We will describe the changes induced in cancer cells upon internalisation of CAF-tracks (Figure 4).
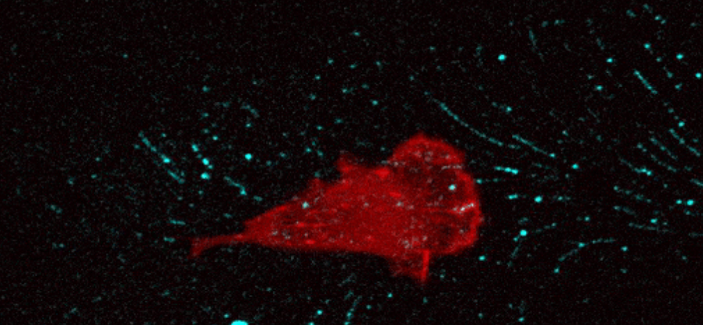
All the modifications occurring in the tumour microenvironment generate a challenging environment for disseminating cancer cells. These cells must establish adhesions to the surrounding environment to evade cell death.. Metastatic cells must therefore exhibit significant plasticity in their adhesive capacities. Our group is studying how metastatic cancer cells switch between different types of adhesions in response to the evolving characteristics of the tumour microenvironment. We are characterising the recently discovered endocytosis-related adhesions. Their role and significance in comparison to the conventional focal adhesions are as yet unclear.
The following members of Pathophysiology are involved: Francesco Baschieri, Francois Tyckaert, Maria Reichhold, Pere Patrón González, Natalia Regina Melo Santos
Pictures
Selected Publications
- Ghannoum S, Fantini D, Zahoor M, Reiterer V, Phuyal S, Leoncio Netto W, Sørensen Ø, Iyer A, Sengupta D, Prasmickaite L, Mælandsmo GM, Köhn-Luque A, Farhan H. A combined experimental-computational approach uncovers a role for the Golgi matrix protein Giantin in breast cancer progression. PLoS Comput Biol. 2023 Apr 17;19(4):e1010995.
- Zahoor M, Dong Y, Preussner M, Reiterer V, Shameen Alam S, Haun M, Horzum U, Frey Y, Hajdu R, Geley S, Cormier-Daire V, Heyd F, Jerome-Majewska LA, Farhan H. The unfolded protein response regulates ER exit sites via SNRPB-dependent RNA splicing and contributes to bone development. EMBO J. 2024 Oct;43(19):4228-4247. PMID: 39160274
- El-Gazzar A, Voraberger B, Rauch F, Mairhofer M, Schmidt K, Guillemyn B, Mitulović G, Reiterer V, Haun M, Mayr MM, Mayr JA, Kimeswenger S, Drews O, Saraff V, Shaw N, Fratzl-Zelman N, Symoens S, Farhan H, Högler W. Bi-allelic mutation in SEC16B alters collagen trafficking and increases ER stress. EMBO Mol Med. 2023 Mar 14:e16834. PMID: 36916446
- Phuyal S, Djaerff E, Le Roux AL, Baker MJ, Fankhauser D, Mahdizadeh SJ, Reiterer V, Parizadeh A, Felder E, Kahlhofer JC, Teis D, Kazanietz MG, Geley S, Eriksson L, Roca-Cusachs P, Farhan H. Mechanical strain stimulates COPII-dependent secretory trafficking via Rac1. EMBO J. Sep 15;41(18):e110596. PMID: 35938214.
- Baschieri F, Illand A, Barbazan J, Zajac O, Henon C, Loew D, Dingli F, Vignjevic DM, Lévêque-Fort S, Montagnac G. Fibroblasts generate topographical cues that steer cancer cell migration. Science Adv. 2023 Aug 18;9(33):eade2120. PMID: 37585527.
Selection of Funding
Hesso Farhan
– FWF: 10.55776/P35832, Rac1 at the Endoplasmic Reticulum Regulates Secretion (2022-2026)
– FWF: 10.55776/FG20, Organelle proteostasis in cellular quiescence and growth (Hesso Farhan is coordinator, 2023-2027)
– FWF: 10.55776/P36600, Role of the spliceosomal subunit SNRPB in ER-proteostasis (2023-2027)
– FWF: 10.55776/PAT1562424, Noncanonical role of IRE1 as a mechanotransducer (2025-2028)
Utku Horzum
– FWF: 10.55776/ESP634, Targeting the early secretory pathway of multiple myeloma (2024-2027)
Veronika Reiterer-Farhan
– FWF: 10.55776/P36925, Pseudophosphatase STYX diversifies F-Box protein function (2023-2027)
Francesco Baschieri
– FWF: 10.55776/PAT4730323, Plasticity and Energy Adaptations in Cancer Cell Migration (2024-2028)
Collaborations
Christian Behrends, LMU Munich, Germany
Liam Holt, New York University Langone Health, USA
Matthias Weiss, University of Bayreuth, Germany
Wolfgang Högler, Kepler University, Linz, Austria
Roded Sharan, University of Tel-Aviv, Israel
Hilal Lashuel, EPFL, Lausanne, Switzerland