Anichstraße 35
A-6020 Innsbruck
Email: irene.virgolini@i-med.ac.at
Website: https://www.nuklearmedizin-innsbruck.com/
Research year
Research Branch (ÖSTAT Classification)
301206, 301904, 302054, 302013, 302070, 302080, 104020, 104015, 301207, 301305
Keywords
neuroendocrine tumour, peptide ligand radionuclide therapy, Peptide receptor radionuclide therapy, Precision Oncology, prostate cancer, radiotracer development, thera(g)nostics, and thyroid cancer
Research Focus
A focus that the Department of Nuclear Medicine has systematically investigated over the past thirty years is the research on radiolabelled ligands for diagnostic and therapeutic purposes —the theragnostic. We are developing various radiopharmaceuticals aimed at different targets for clinical applications. The primary clinical goal is to introduce more effective ligands, peptides and antibodies for personalised treatment, particularly radiolabelled theragnostic for precision oncology.
General Facts
One focus of the Department of Nuclear Medicine is on imaging biomarkers utilised in cancer treatment (70% of routine clinical practice), addressing neurological impairment (20% of routine clinical practice) and dealing with cardiac and other diseases (10% of routine clinical practice). The main focus in the transition of preclinical research on radiopharmaceuticals (specifically radiolabelled peptides) into clinical applications is found in tumour imaging, infection imaging and imaging of functional liver mass. The department is centred on a state-of-the-art preclinical research and development unit that is well-funded and comprises radio-pharmacists/chemists, biologists, medical physicists and PhD students.
In dedicated laboratories, radiopharmaceuticals for SPECT/CT or PET/CT studies are produced. These radiotracers utilise various modal systems, including a broad range of radiolabelled peptide analogues such as those for somatostatin receptor (SSTR), fibroblast activation protein inhibitor (FAPi), cholecystokinin (CCK-2/gastrin), chemokine receptor type 4 (CXCR4) and prostate-specific membrane antigen (PSMA) for precise tumour targeting. Other significant advancements include Arg-Gly-Asp (RGD) for imaging angiogenesis in tumour lesions and asialoglycoprotein receptor imaging with galactosylated albumin (GSA) and derivatives to assess the functional liver reserve.
The team of the PET centre and the conventional nuclear medicine clinic conducted approximately 40 clinical trials in cooperation with different other clinics. The department is involved not only in many clinical studies with radiolabelled theragnostic, particularly industry-sponsored Phase I/IIa trials, but also in academic phase I trials with radiopharmaceuticals developed in-house. The nuclear medicine therapy ward provides treatment to patients who have been initially evaluated through SPECT/CT dosimetry studies, using high-dose radiolabelled theragnostic. Now, the most important therapy tools are radioiodine ablation therapy of thyroid cancer remnants, peptide receptor radionuclide therapy (PRRT) of neuroendocrine tumour (NET) patients and prostate specific membrane antigen (PSMA) ligand radionuclide therapy (LRT) of prostate cancer patients.
Research
The research efforts of the Department of Nuclear Medicine concentrate on preclinical studies aimed at refining and enhancing radiolabelling methods for known radiopharmaceuticals, preparing new radiopharmaceuticals for clinical trials and developing new radioligands for molecular imaging and therapy. Various research projects exemplify the work in this area. Several of these theragnostics have progressed to larger Phase I/II clinical trials.
-
Siderophores for Infection Imaging
Radio-pharmacy Lead: Clemens DECRISTOFORO
Clinical Translation: Gianpaolo Di SANTO, Bernhard NILICA, Mailin MANN (MD Thesis)
Nature has designed siderophores, produced by bacteria, fungi and plants to bind ferric ions, thereby providing a competitive advantage in an essentially iron depleted environment. In recent years our group has concentrated on developing siderophore-based probes for PET imaging of infection using radiolabelled natural siderophores, thereby replacing iron by Gallium-68 with almost identical coordination chemistry as ferric iron (Ref.1).
In a current project (SIDEROART), started in 2024 and funded by the FWF (I66613-B) under the WEAVE programme, we focus on synthetic biomimetic analogues of natural hydroxamate siderophores to reveal their potential as the basis for novel non-invasive in-vivo imaging agents. Our preliminary investigations showed excellent properties of artificial, 68Ga-labelled ferrioxamine biomimetics for infection imaging. Modification of these candidates allows development of multifunctional chelators for multimodal or multivalent agents. This main objective, i.e. the design and development of novel non-invasive in-vivo imaging agents, biomimetics for the internalisation of ferric-siderophores and possibly drug delivery systems, will be achieved through an interdisciplinary approach that combines the unique expertise of four research groups located in Poland, the Czech Republic and Austria.
The successful preclinical development of Gallium-68 siderophores for infection imaging via PET has been translated into clinical practice. A Phase I/IIa clinical trial with the clinically approved drug deferoxamin (Desferal®) labelled with Gallium-68 for the imaging of bacterial infections was approved (EudraCT No: 2020-002868-31) in 2021, supported by the FWF within the KLIF-track (Project No. KLI 909-B) and was successfully completed with the enrolment of 14 patients at the end of 2024. For the first time, the clinical translation shows the proof-of-concept of targeting infections with radiolabelled siderophores: [68Ga]Ga-deferoxamine was safely applied to patients with different kinds of infections and demonstrated infectious foci by PET imaging (Fig.1)
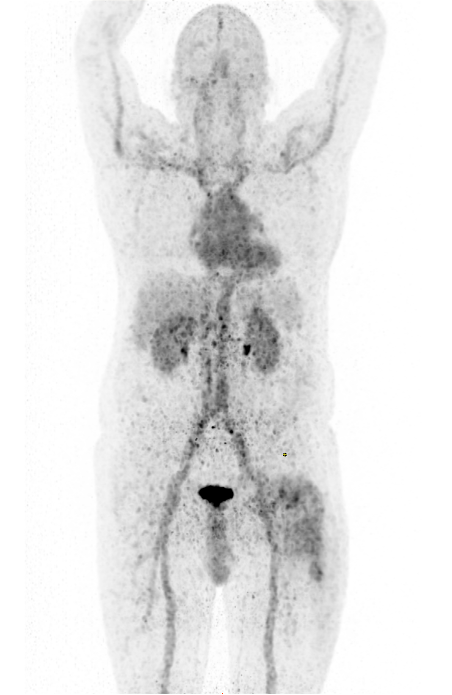
Imaging of infection in the area of the hip endoprosthesis using [68Ga]Ga-Desferoxamine demonstrating moderate uptake in the left thigh two hours post-injection.
-
Development of Multimodality Imaging Probes
Clemens DECRISTOFORO & Giacomo GARIGLIO (PhD Student)
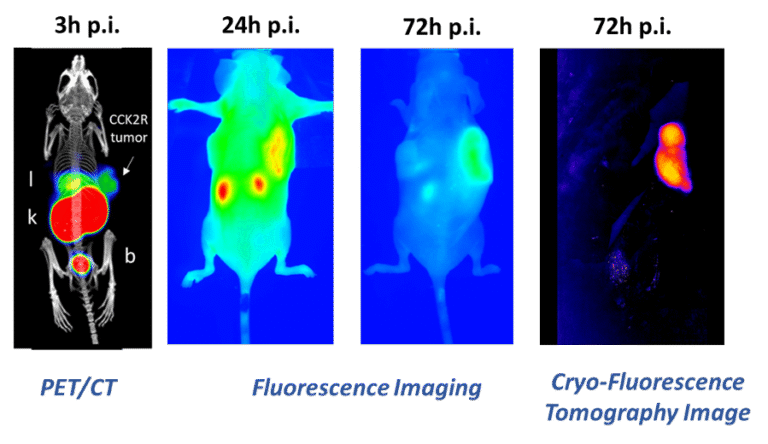
The complete resection of solid tumours remains challenging and end-of-surgery positive surgical margins are common especially in case of ovarian, prostate and head & neck cancer. Dual-modality probes, combining PET with fluorescence imaging (FI) capabilities in the same molecule, can be used for both preoperative imaging and intraoperative real-time guidance, and can therefore improve the outcome of surgery.
Within the Doc-Funds Project IGDT-ART, funded by the FWF, novel PET/FI probes are developed using a chelator scaffold, to which a specific tumour-targeting sequence and a near-infrared (NIR) fluorescent dye suitable for FI are attached. First results showed that both Fusarinine C, a hydroxamate siderophore, and TRAP, a synthetic cyclic chelator, serve as effective scaffolds for developing such probes, which can be easily labelled with Gallium-68 for PET imaging. Initial development aimed at targeting CCK2 receptors, was hindered by suboptimal pharmacokinetics at early time points due to the lipophilic nature of both the applied dyes and the targeting sequence. However, almost exclusive tumour retention was found at later time points (Fig.2; Ref.2).
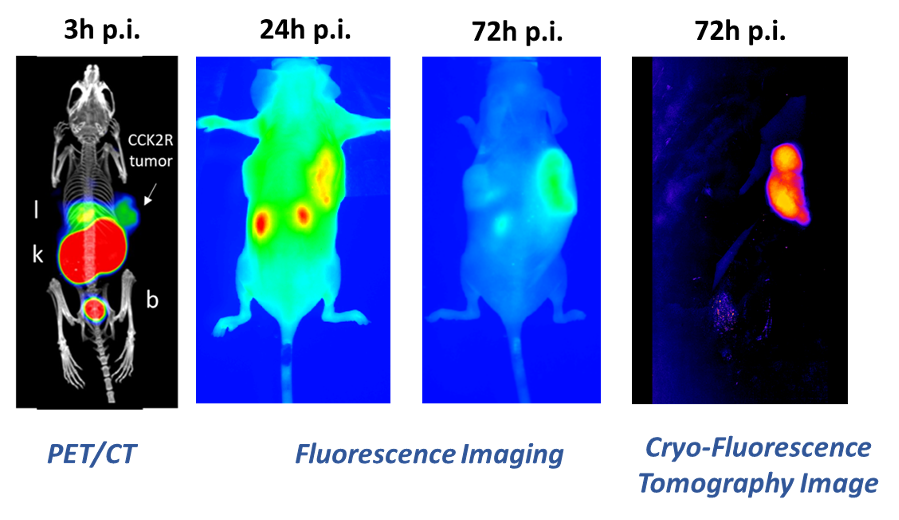
-
Cholecystokinin Receptor (CCK2R) Theragnostics
Radiopharmacy Lead: Elisabeth von GUGGENBERG & Taraneh ZAVVAR (PhD Student)
Clinical Translation: Christian Uprimny, Gianpaolo DI SANTO, Giulia SANTO (PhD Student), Lisa-Maria Rossetti, Steffen Bayerschmidt, Christine Rangger, Irene Virgolini
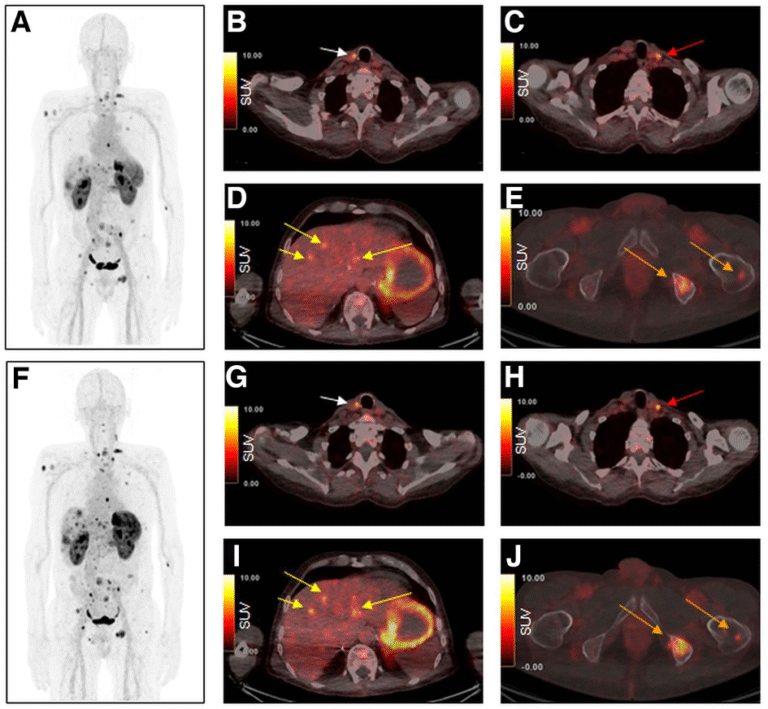
Radiolabelled minigastrin (MG) analogues specifically target the CCK2R, a peptide receptor over-expressed in various tumour types, including medullary thyroid carcinoma (MTC), small cell lung cancer (SCLC), as well as gastroenteropancreatic and bronchopulmonary NETs. A number of radiolabelled MG analogues have been developed over the past twenty years, but none has found wider clinical application due to high kidney uptake or low stability in-vivo. The most promising candidate of our developments was DOTA-MGS5, a short peptide sequence of eight amino acids conjugated to the macrocyclic chelator DOTA, which enables radiolabelling with various trivalent radiometals (Ref.3). In preclinical tumour animal models, DOTA-MGS5 radiolabelled with Gallium-68 or Lutetium-177 showed high and persistent tumour uptake, combined with favourable pharmacokinetics (Ref.4). Throughout the last years, the clinical translation of PET/CT imaging with [68Ga]Ga-DOTA-MGS5 was pursued. From 2021-2023, a prospective Phase I/IIa trial was conducted to evaluate the safety and preliminary diagnostic performance of [68Ga]Ga-DOTA-MGS5 (ClinicalTrials.gov: NCT06155994). Six patients with advanced MTC and six patients with gastroenteropancreatic and bronchopulmonary NETs were included in the study (Ref.5). In four patients with MTC, a patient with ileal NET and two patients with lung NET [68Ga]Ga-DOTA-MGS5-avid lesions known from reference PET/CT imaging with [18F]F-DOPA or [68Ga]Ga-SST analogue were confirmed to show favourable safety and biodistribution (Fig.3).
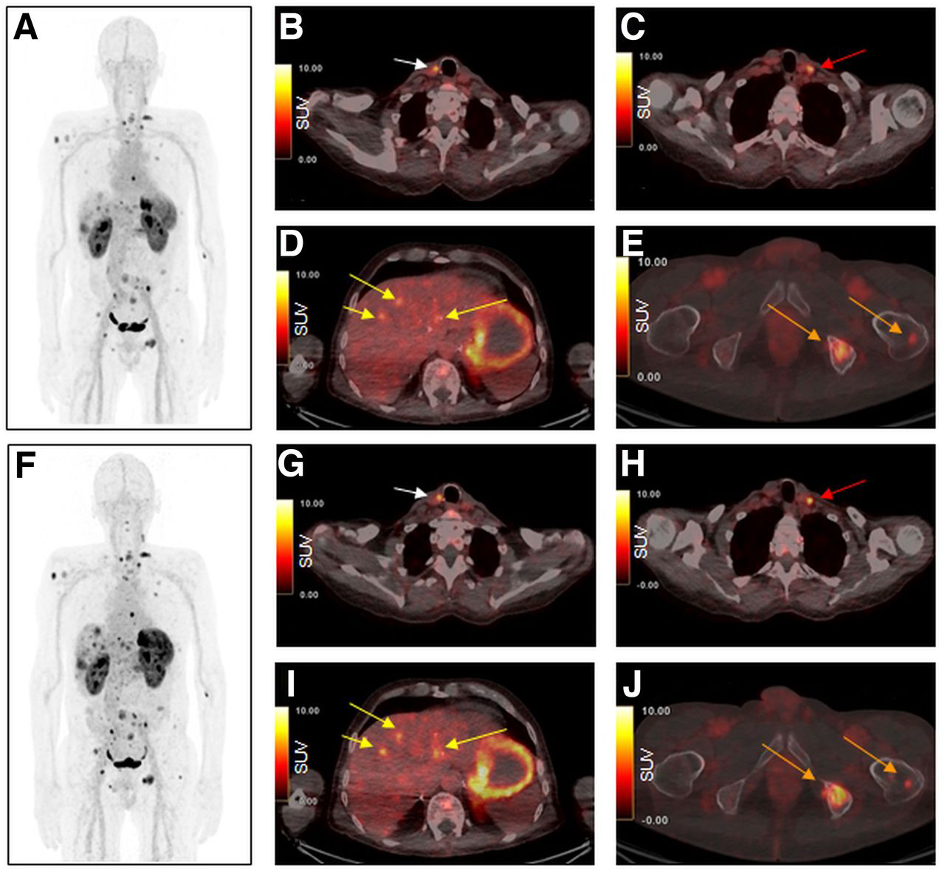
Maximum-intensity projection and axial PET/CT images 1 h (A–E) and 2 h (F–J) after injection of [68Ga]Ga-DOTA-MGS5. Images show local recurrence in right paratracheal region (B and G; arrows), a cervical lymph node metastasis is seen in left cervical region (C and H; arrows), liver metastases (D and I; arrows) as well as bone metastases in left iliac and left femur (E and J; arrows). Images reproduced from von Guggenberg et al. JNM 2023; 64: 859-862 (Ref.4).
As part of this project, further optimisation of the peptide sequence and alternative radiolabelling strategies with other positron emitters are being investigated. First steps in the clinical translation of peptide receptor radionuclide therapy with DOTA-MGS5 radiolabelled with the beta emitting radionuclide [177Lu]Lu-DOTA-MGS5 could be reached recently within a project of the FWF funded doctoral programme “IGDT – integrating multimodal strategies for clinical research” (FWF DOC 110 doc.funds; grant DOI 10.55776/DOC110; Ref.6). The successful validation of the radiopharmaceutical formulation for clinical application enabled the first dosimetry study with [177Lu]Lu-DOTA-MGS5 in a patient with SCLC (Ref.7). PET/CT imaging with [68Ga]Ga-DOTA-MGS5 in this patient revealed additional abnormal foci, in addition to those with high [18F]F-FDG uptake, while SSTR expression was low, as shown by weak [68Ga]Ga-SST analogue uptake (Fig.4).
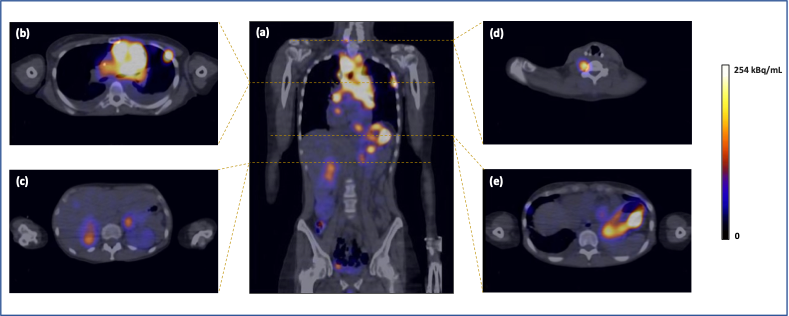
A major success was the establishment of a cooperation between the Medical University of Innsbruck and Evergreen Theragnostics in the USA, which has licensed the technology in 2023. New Phase 1 clinical trials with 68Ga-DOTA-MGS5 (EUCT number 2024-514584-25-00) and 177Lu-DOTA-MGS5 (EUCT number 2024-518039-12-00) are currently being initiated in close cooperation with the industrial partner in order to evaluate this new theragnostic approach in patients with SCLC first, and in MTC next.
-
Angiogenesis Imaging – Integrin αvβ3 Expression Theragnostics
Radiopharmacy Lead: Roland HAUBNER
Clinical Translation: Gianpaolo Di SANTO, Stephan ZIRKNER (MD Thesis), Irene VIRGOLINI
A key process in the development of cancer is tumour-induced angiogenesis. An important receptor involved in this complex process is the integrin αvβ3. As part of the project, a Gallium-labelled small cyclic peptide, namely [68Ga]Ga-NODAGA-RGD, was developed. Based on the promising preclinical results and an initial clinical study demonstrating safety and tolerability of the compound, a Phase 1 study was initiated to investigate the potential of this new radiopharmaceutical (EudraCT-No. 2018-001016-30) in patients with breast cancer, glioblastoma, renal cell cancer, non-small-cell lung cancer or NETs. The clinical trial was stopped at the end of 2023 due to a series of negative findings in the first patients with NETs as well as the Covid-19 pandemic, which led to recruitment problems.
-
New PET Tracer for Imaging the Functional Liver Reserve
Radiopharmacy Lead: Roland HAUBNER Members: Maximilian ZIERKE (PhD Student),
Clinical Translation: Fabian Scherbauer, Christine RANGGER, Irene VIRGOLINI
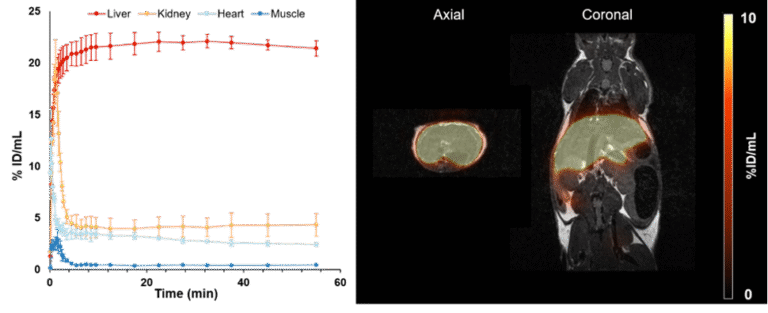
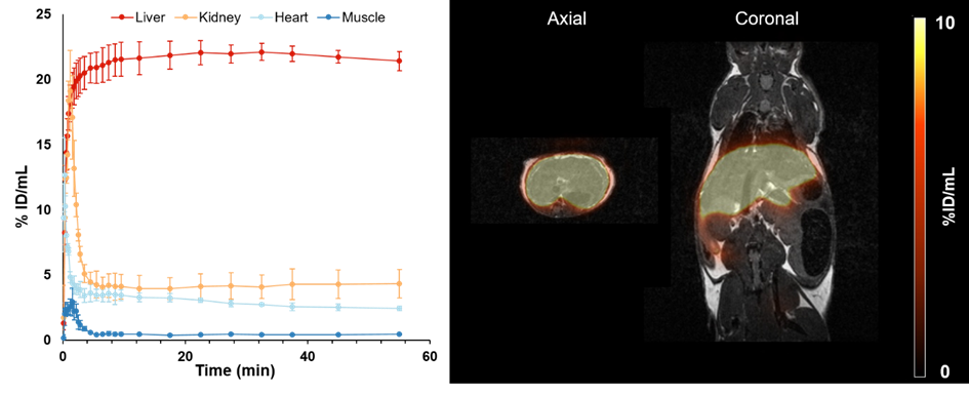
The asialoglycoprotein receptor (ASGR) is a C-type lectin mainly expressed on the basolateral side of hepatocytes, where up to 500,000 receptors per cell can be found. In contrast, its expression in the rest of the body is low, making it a promising target for drug delivery into hepatocytes and an optimal target structure for non-invasive monitoring of the functional liver reserve. The initial developments of this project resulted in [68Ga]Ga-NOTA-GSA, which demonstrated high metabolic stability and confirmed the superiority of PET for imaging the functional liver reserve (Ref.8). Despite the good imaging performance, the translation of this human serum albumin-based labelled precursor into clinical practice was not possible. To satisfy these requirements, the development of new derivatives has begun, and a corresponding proposal is now funded by the FWF (P 34802-B). Meanwhile, this project resulted in two different classes of low molecular weight derivatives of the GSA, namely organic compounds presenting three galactose moieties and peptides containing between three and nine galactose moieties. Both classes can be labelled with Gallium-68. Among these sets, two compounds have been identified that possess even better imaging properties than the lead compound [68Ga]Ga-NOTA-GSA (Fig.5; Ref.9).
- Novel Radionuclides for Theragnostic Applications (PRISMAP Project)
Clemens DECRISTOFORO & Hessamoddin Roustaei FIROUZABAD
To provide the most extensive catalogue of radionuclides for medical research, a network of top-tier European facilities has been established, comprising nuclear reactors, medium- and high-energy accelerators and radiochemical laboratories. The advancement of increasing the production of these new radionuclides will be explored through innovative production technologies, improved purification techniques and proof-of-concept studies, demonstrating the transition of new treatments from test bench to patient care. PRISMAP (www.prismap.eu, funded by the Horizon 2020 research and innovation programme, grant agreement No 101008571) strives to create a paradigm shift in the early-phase research on radiopharmaceuticals, targeted drugs for cancer theragnostics and personalised medicine. Work package 4 of the PRISMAP project, led by the Department of Nuclear Medicine, initiated several activities to harmonise the pharmaceutical standards required for clinical translation of innovative radionuclides. New guidelines and recommendations were initiated for both quality and safety standards of novel radionuclides for medical applications, thereby involving radionuclide research centres, clinical centres and pharmaceutical regulators throughout Europe to support the research in novel radiopharmaceuticals, the project will finish in 2025.
-
Novel Technetium-99m labelled Somatostatin Antagonists – TECANT
Radiopharmacy Lead: Clemens DECRISTOFORO
Clinical Translation: Gianpaolo DI SANTO, Christine RANGGER, Irene VIRGOLINI
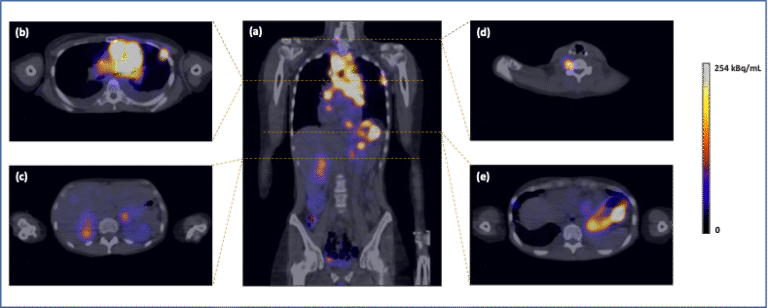
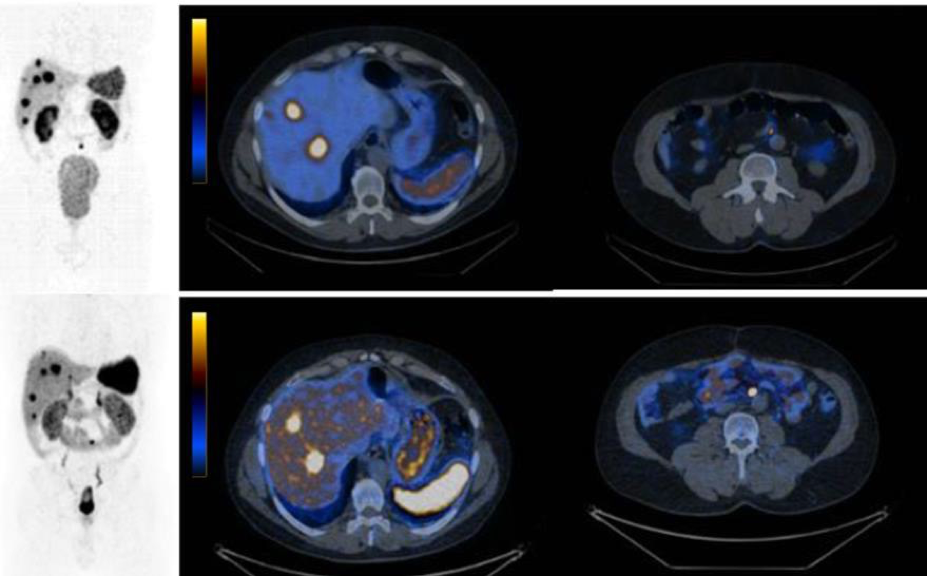
The Era-PERMED TECANT project [https://www.era-learn.eu/network- information/networks/era-permed/1st-joint-transnational-call-for-proposals-2018/] was a collaboration between the University of Krakow in Poland, the University of Ljubljana in Slovenia and the University of Basel in Switzerland. The major aim of the project was the development of a receptor antagonist labelled with Technetium-99m for SPECT imaging. In the initial experimental phase, the antagonist [99mTc]Tc-TECANT1 showed better targeting properties for NETs and was selected for the clinical study (Phase 0/I clinical trial; EudraCT no:2019-003379-20). [99mTc]Tc-TECANT1 SPECT/CT imaging was performed in all scheduled 10 patients; no side effects were observed and an excellent visualisation of NET lesions was obtained in most cases with tumour-to-background ratios (TBRs) superior to [68Ga]Ga-SSTR agonists. T/B ratios for SPECT/CT were even higher than those for PET/CT, with several lesions not even detected by PET imaging (Fig.6).
-
68Ga-labelled Bombesin Antagonist Theragnostics (NeoRAY)
Radiopharmacy Lead: Clemens DECRISTOFORO
Clinical Translation: Bernhard NILICA, Gianpaolo DI SANTO, Christine RANGGER, Irene VIRGOLINI
As early as in 2016, the Department of Nuclear Medicine conducted the first diagnostic in–vivo study with a novel [68Ga]Ga-labelled Bombesin antagonist, a GRPR antagonist, for PET in patients with gastrointestinal stromal tumours (GIST) (EU FP7 project MITIGATE). In the subsequent NeoFIND study (NCT03724253), the diagnostic performance of [68Ga]Ga-NeoB was investigated in order to detect GRPR-positive tumour lesions. The [68Ga]Ga-NeoB study included 19 patients with solid tumours (including 5 breast cancer patients) and provided the basis for the multi-centre, multi-national clinical Phase 3 trial “NeoRAY”, which was completed in Innsbruck by the end of 2024). In the NeoRAY study, patients with solid tumours were first scanned by [68Ga]Ga-NeoB PET/CT for tumour detection and, after central review of the images in the US, treated with [177Lu]Lu-NeoB in an escalating manner typical for Phase 1 studies (EudraCT 2018-004727-37). Study results are currently evaluated and will be presented by NOVARTIS in due time (with the main recruiting centre at Stanford, USA).
-
PSMA (Prostate-specific Membrane Antigen) Theragnostics
Clinical Lead: Irene VIRGOLINI
Members: Gianpaolo Di SANTO, Giulia SANTO, Christian Uprimny, Christine RANGGER
Radiopharmacy: Clemens DECRISTOFORO, Elisabeth von GUGGENBERG, Emanuel SPORER
Based on the over-expression of PSMA on prostate cancer (PC) cells, the Gallium 68-labelled PSMA ligand HBED-CC has proven its feasibility for the detection of PC relapses and metastases with high sensitivity. Our recent multicentre retrospective analysis for patients with PSA relapse has shown significant differences in overall survival for patient groups with PSA <0.2, 0.2-0.5, 0.51-1.0 and >1.0 ng/ml before restaging with [68Ga]Ga-PSMA-PET/CT (Ref .11), demonstrating also the significant role of [68Ga]Ga-PSMA-PET/CT scan results for patient management that has emerged over the past decade (Ref.12; Ref.13). Furthermore, the Department of Nuclear Medicine was part of the multicentre academic prospective trial (EudraCT 2016-001815-19) that resulted in the implementation in recent guidelines for the work-up of PC patients.
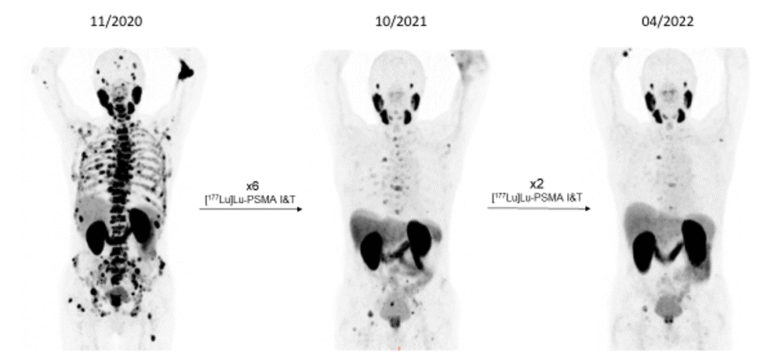
Based on the high-level expression of PSMA on PC cells, high-dose 177Lu-PSMA ligands (in Innsbruck PSMA I&T; PSMA617) were used to treat PC patients with metastasised diseases.
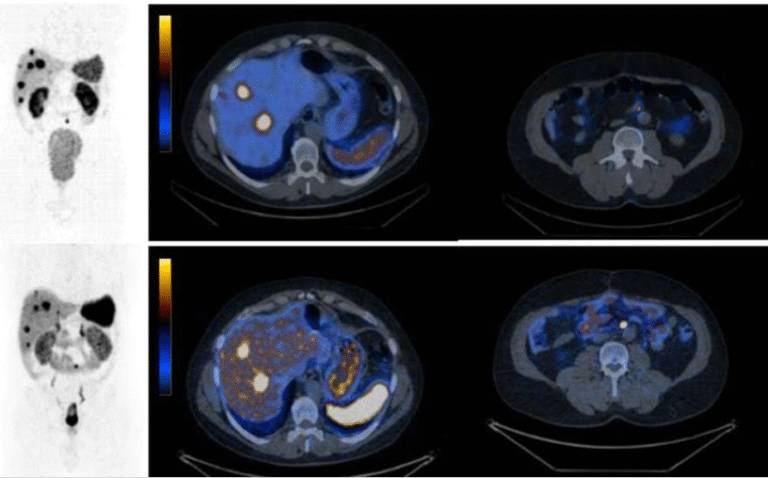
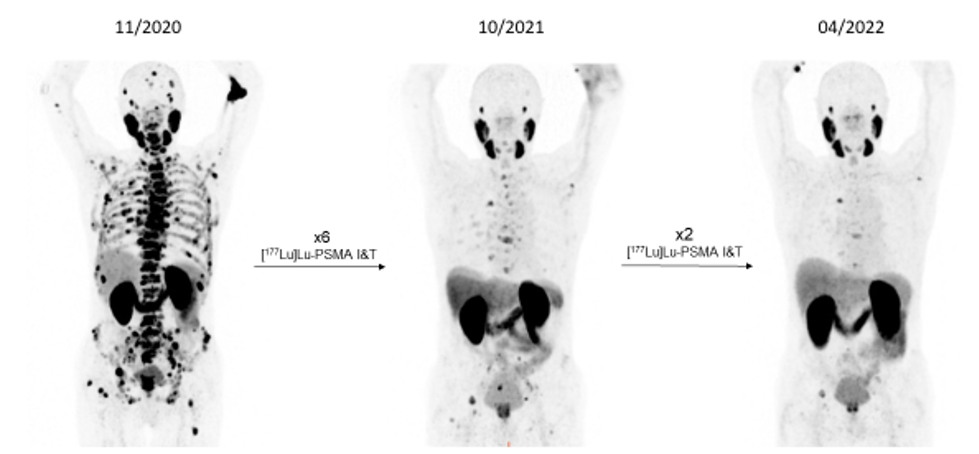
Results throughout recent years demonstrate high tumour control ability of these radiopharmaceuticals with significant implications for future PC therapy protocols, especial evidenced by the prospective VISION study results (NOVARTIS). WARMTH (World Association of Radiopharmaceutical and Molecular Therapy)-sponsored retrospective multicentre trials, partly approved by Innsbruck Ethics Committee, showed significant results superior to chemotherapy – results only later supported by the prospective THERAP study published by Australian Centres. Combination schedules and new radioligands as well as mixtures of radioligands for the treatment of PC are currently being discussed. In our Innsbruck cohort of PC patients, we demonstrated the advantages of re-challenging the PSMA therapy for newly progressed mCRPC patients (as confirmed by [68Ga]Ga-PSMA-11 PET/CT) after a response to the initial [177Lu]Lu-PSMA RLT [Fig. 7).
The industry-sponsored clinical study in collaboration with our Urology Department PSMAfore (A phase III, Open-label, Multi-Centre, Randomised Study Comparing [177Lu]Lu-PSMA-617 vs. a Change of Androgen Receptor-directed Therapy in the Treatment of Taxane Naïve Men with Progressive Metastatic Castrate Resistant Prostate Cancer (CAAA617B12302)) was clearly in favour of PSMA-LRT. The PSMAddition trial (An International Prospective Open-label, randomised, Phase III Study comparing [177Lu]Lu-PSMA-617 in Combination with Standard of Care, versus Standard of Care alone, in Adult Male Patients with Metastatic Hormone Sensitive Prostate Cancer (mHSPC) (CAAA617C12301)) has closed recruitment and results are soon going to be presented by NOVARTIS.
Furthermore, a significant role of α-emitters coupled to targeting agents, such small molecule ligands, peptides or antibodies, is emerging due to their shorter travel path (40–100 µm), which is perfect for DNA double strand breaks – thus α-particles hold great promise for enhancing tumour response. 225-Actinium-labelled PSMA ligands are already in use for progressed PC patients who have undergone chemotherapy, an androgen receptor pathway inhibitor or a course of [177Lu]Lu-PSMA ligand therapy. The Novartis sponsored clinical trial PSMAction trial (NCT 06780670) with Actinium-225-labelled PSMA617) is set to start recruitment at Innsbruck in January 2026. Due to the world-wide shortage of Actinium-225, our team has started to set up radiopharmaceutical development of a new treatment strategy using Terbium-161-labelled alternatives.
Somatostatin Receptor Theragnostics
Clinical Lead: Irene VIRGOLINI
Members: Gianpaolo DI SANTO, Giulia SANTO, Margarida RODRIGUES, †Anna SVIRIDENKO
Peptide receptor radionuclide therapy (PRRT) for gastroenteropancreatic NETs currently follows a standardised treatment schedule using [177Lu]Lu-DOTATATE, administered in four cycles (7.4 GBq each, eight weeks apart) alongside amino acid infusion to reduce kidney radiation exposure. The latest focus guideline from the European Association of Nuclear Medicine (EANM) suggests PRRT as a first-line treatment for non-resectable/disseminated NET in a small number of carefully selected patients with high somatostatin receptor (SSTR) expression. It also suggests PRRT as a second-line treatment for gastroenteropancreatic neuroendocrine tumours (GEP-NETs) if sufficient uptake in all lesions is demonstrated. Consideration should also be given to PRRT for GEP-NET patients at the first sign of disease progression, provided that all matched lesions are [68Ga]Ga-DOTA-SST analogue/18F-FDG positive and Ki67 is less than 20% (G1 and G2), or in a minority of patients with G3 Ki67 greater than 20%.
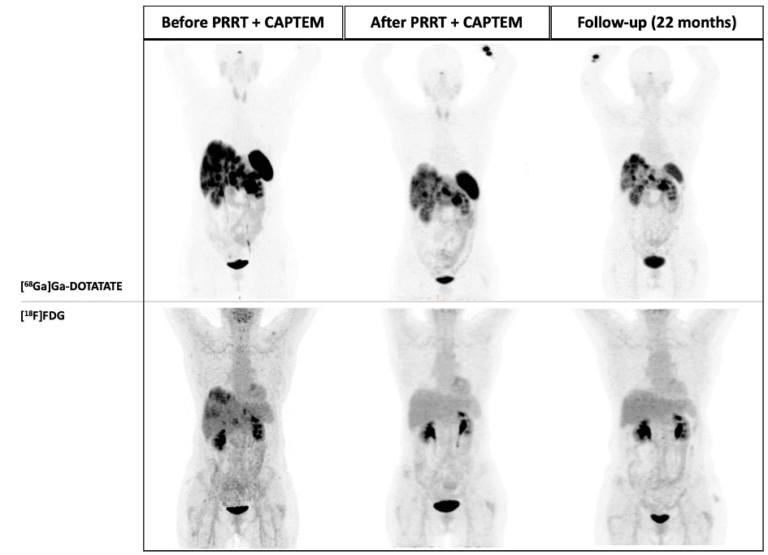
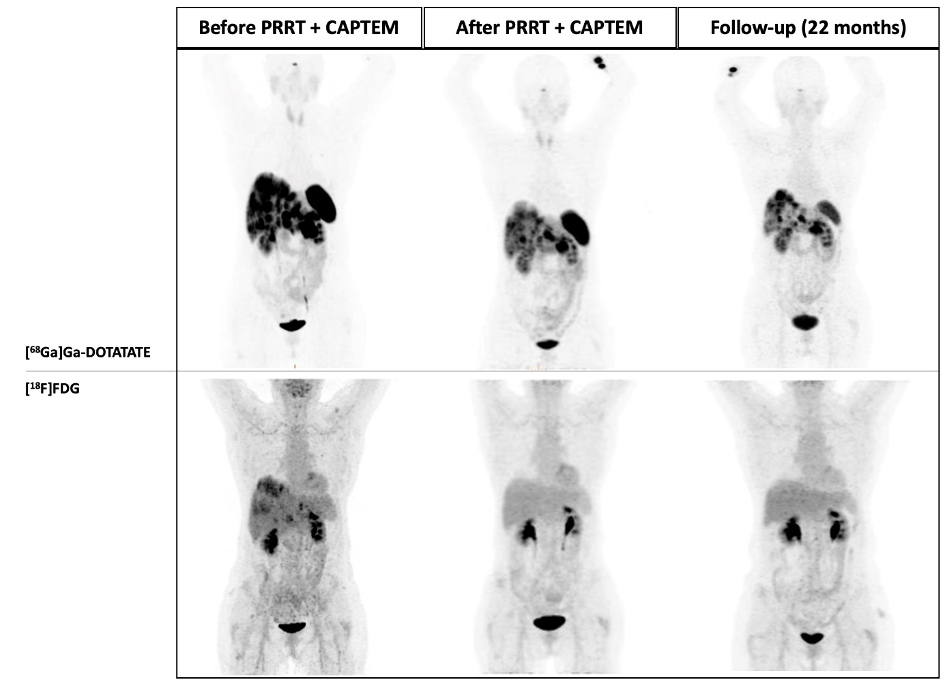
In recent years, the efforts made to enhance PRRT results substantially relied on the understanding of intra- and inter- tumour heterogeneity and the ability of a tailored personalised approach to NET patients. Specifically, dual tracer [68Ga]Ga-DOTA-SSA / [18F]F-FDG PET/CT imaging has been shown to provide a more comprehensive view of the tumour landscape in NET patients, potentially overcoming limitations associated with single-site biopsies and improving our understanding of disease variability. In clinical practice, highly SSTR-avid (G1/G2) NETs are usually treated with long-acting SST analogue followed by PRRT, whereas highly FDG-avid with low SSTR-expression (i.e., G3) NETs are commonly treated with chemotherapy. However, some NETs patients exhibit a high uptake on both [68Ga]Ga-DOTA-SSTR and [18F]F-FDG PET/CTs. For these patients, a therapeutic approach combining PRRT with chemotherapy may present an effective strategy (Fig.8).
Neurotensin Receptor Theragnostics
In collaboration with Professor Feng Wang from Nanjing in China (where Irene Virgolini is an appointed Visiting Professor), initial [68Ga]Ga-labelled neurotensin analogue-PET/CT scans were performed in patients with exocrine pancreatic cancer in Nanjing. The positive neurotensin scans acquired suggests a potential future role for such analogues, as well as the possibility of identifying a more stable [177Lu]Lu-labelled neurotensin analogue for the treatment of pancreatic cancer.
Fibroblast Activation Protein (FAP) Inhibitor Imaging
Lead Radiopharmacist: Clemens DeCristoforo
Clinical translation: †Anna SVIRIDENKO & Irene VIRGOLINI
In recent years, imaging FAP-expression on fibroblasts has become a useful tool for oncology patients using [68Ga]Ga-labelled inhibitors. We used [68Ga]Ga-FAPI-46 to monitor SARS-COV2-infected patients. Exaggerated fibrotic responses or excessive inflammation can lead to tissue damage in non-malignant human diseases. There are substantial differences between the molecular and cellular basis of these two processes, their influence on the prognosis of disease and the notion of treatment. As a result, it is highly desirable to evaluate and quantify these two processes in-vivo simultaneously. The evaluation of the molecular dynamics of fibrosis is still difficult, despite the fact that non-invasive molecular techniques such as [18F]F-FDG-PET provide information on the level of inflammatory activity. Patients with combined fibro-inflammatory pathology and chronic CT abnormalities following a severe case of COVID-19 may benefit from the non-invasive clinical diagnostic performance of the [68Ga]Ga-FAPI-46. First studies indicated a potential role for FAP-Imaging in impaired pulmonary convalescence (Ref. 22).
Chemokine Receptor Type 4 (CXCR4) Theragnostics
Lead Radiopharmacist: Clemens DeCristoforo
Clinical translation: Lisa Maria Rossetti, Lilit Schweiger, Giulia SANTO, Gianpaolo Di Santo, Irene VIRGOLINI
CXCR4 plays an essential role during embryonic organogenesis and orchestrates key immunological functions as a crucial regulator of leukocyte migration. Furthermore, CXCR4 is key in different cancer types. Many different malignancies overexpress CXCR4 on their cell surfaces, including solid cancers (e.g., breast or prostate cancer) and haematological malignancies (e.g., leukaemia or lymphoma). In the ongoing clinical trial (EUCT No. 2022-500918-25-00) [68Ga]Ga-PentixaFor (PTF) is applied for PET/CT imaging for the primary staging of patients with histologically confirmed marginal zone lymphoma (MZL). [68Ga]Ga-(PTF) is a peptide CXCL12 analogue that binds to CXCR4 receptors.
Other research activities using [68Ga]Ga-PTF PET scans to demonstrate CXCR4 expression are currently under way in patients with rare tumours, in order to assess CXCR4-targeted therapy option with [177Lu]Lu-PTF ligand for individualised treatment approaches (Fig.9).
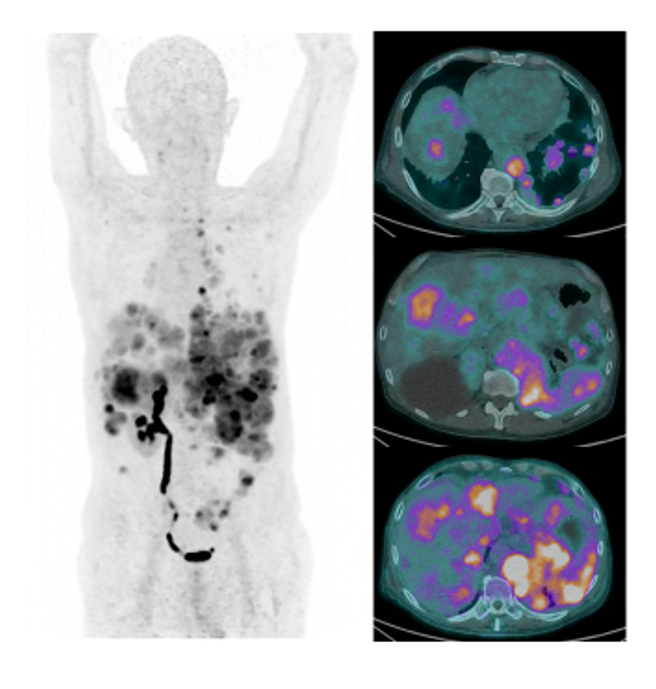
Glucagon Like (GL)-1 Receptors (Exendin)
Clinical Translation: Irene Virgolini & Lilit Schweiger
In patients with clinical symptoms of insulinoma (leading symptom hypoglycaemia due to elevated insulin production leading to dizziness, tachycardia, tremor, etc.) the localisation of the suspected pancreatic tumour is limited using conventional means (ultrasound 40%, CT 60%, MRT 50%). In recent years [68Ga]Ga-NOTA-exendin-4 (Ref.23), which specifically binds to GLP-1 receptors overexpressed on insulinoma cells, has gained clinical interest as these tumours are frequently negative on [68Ga]Ga-DOTA SSTR PET/CT or MR scans. We have recently adopted the methodology for clinical use in patients with suspected insulinoma (Fig.10).
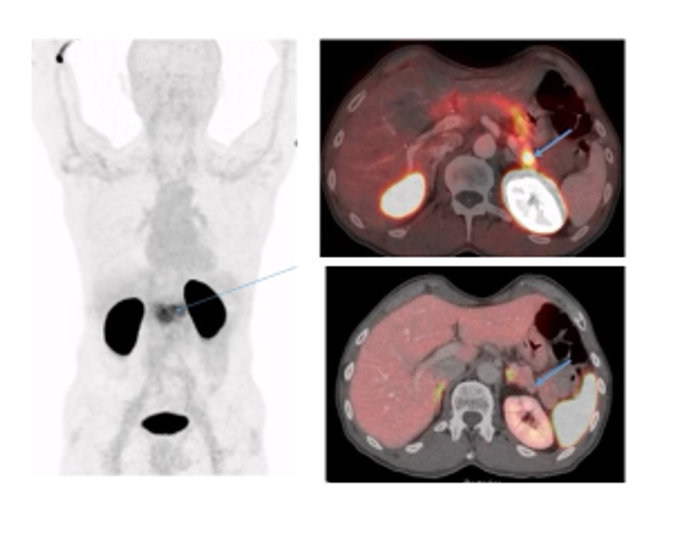
After injection of [68Ga]Ga-DOTA-Exendin-4 in a 62 year-old patient with suspected insulinoma PET/CT images show the physiological biodistribution with low to moderate accumulation in the normal pancreatic tissue (left: maximal intensity projection) and the suspected small-sized tumour lesion detected by a high focal uptake pattern (right upper panel) with negative CT (right lower panel). Surgery and pathohistology confirmed the accumulation of the radiotracer in the insulinoma.
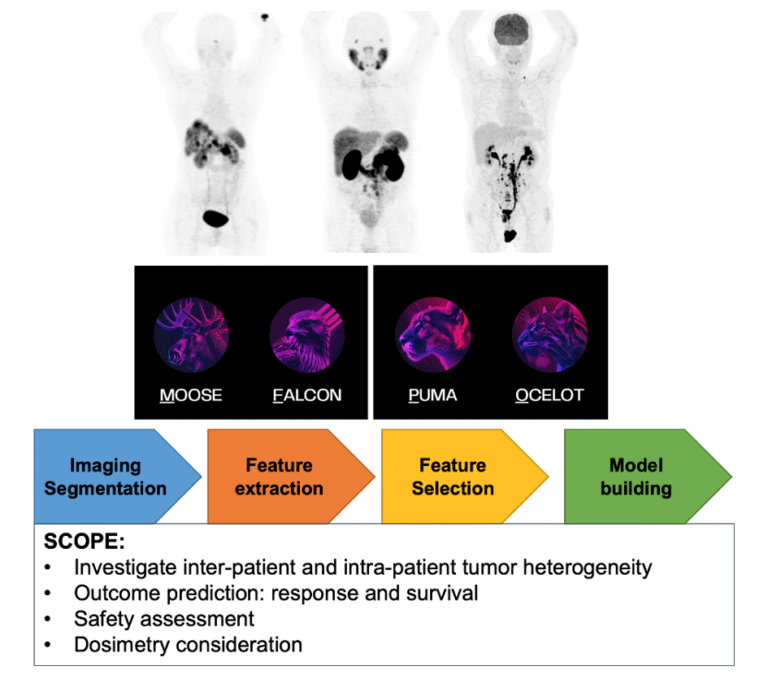
Nuclear Medicine and Artificial Intelligence
Giulia SANTO & Ariane KROHNTHALER
Technological progress and the gradual introduction of artificial intelligence into clinical practice are revolutionising the way images are being analysed. We have proposed a protocol for the retrospective study of PET/CT and SPECT/CT image analysis for the exploration of theragnostic applications. The investigations employ a range of analytical methods based on classical statistics, machine learning and radiomics, with the aim of supporting future studies using Nuclear Medicine imaging data (Fig.11).
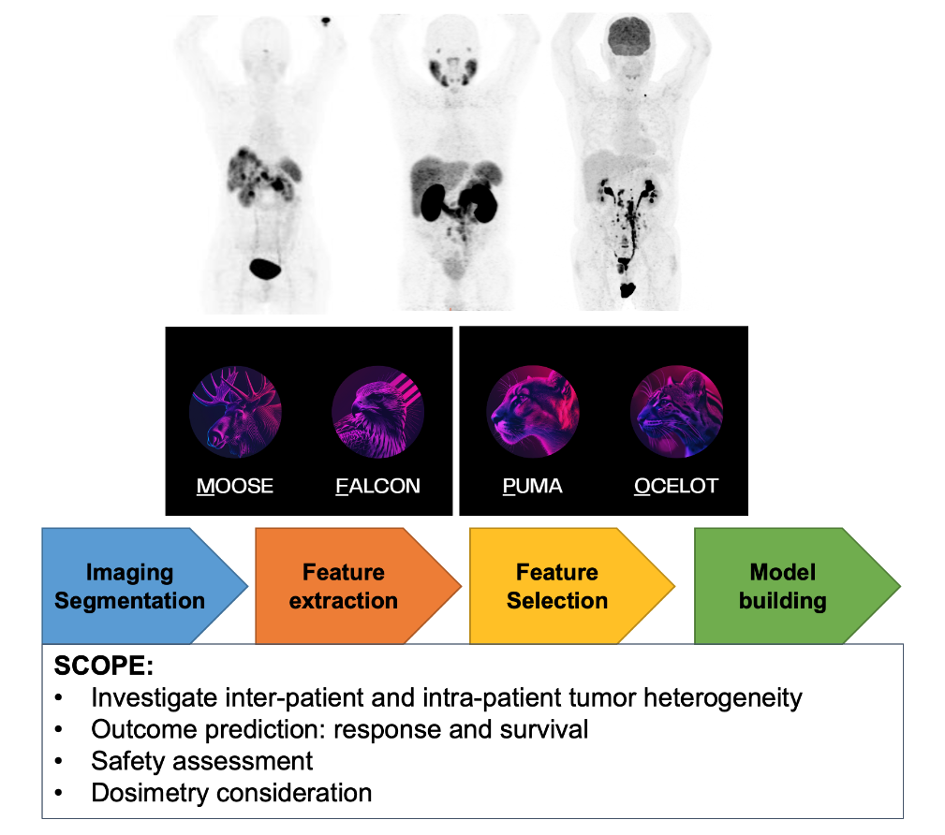
In this context, open-source software tools from the ENHANCE-PET framework developed by the QIMP team of the Medical University of Vienna (available at https://enhance.pet) will be used to analyse the images (Protocol number: 1296/2024 Title: “Retrospektive Bildanalyse für “Thera(g)nostik””).
Pictures
Selected Publications
- Defrancesco M, Gizewski ER, Mangesius S, Galijasevic M, Virgolini I, Kroiss A, Marksteiner J, Jehle J, Doganyigit B, Hofer A. Investigating patient eligibility for anti-amyloid monoclonal antibody treatment of Alzheimer’s disease: real-world data from an Austrian psychiatric memory clinic population. BJPsych Open. 2024 Sep 23;10(5):e160. doi: 10.1192/bjo.2024.747.
- Kraihammer, M., Petřík, M., Rangger, C., Gabriel, M., Haas, H., Nilica, B., Virgolini, I., & Decristoforo, C. Automated production of [68Ga]Ga-Desferrioxamine B on two different synthesis platforms. PHARMACEUTICS, 2024;16(9):1231.
- Gariglio, G., Bendova, K., Hermann, M., Olafsdottir, A., Sosabowski, J. K., Petrik, M., von Guggenberg, E., & Decristoforo, C. Comparison of two chelator scaffolds as basis for cholecystokinin-2 receptor targeting bimodal imaging probes. PHARMACEUTICALS, 2024;17(12):1569.
- Hoermann, A. A., Klingler, M., Rangger, C., Mair, C., Joosten, L., Franssen, G. M., Laverman, P., & von Guggenberg, E. Effect of N-terminal peptide modifications on in vitro and in vivo properties of 177Lu-labeled peptide analogs targeting CCK2R. PHARMACEUTICS, 2023;15(3):796.
- von Guggenberg, E., Uprimny, C., Klinger, M., Warwitz, B., Sviridenko, A., Bayerschmidt, S., di Santo, G., & Virgolini, I. J. Preliminary clinical experience with cholecystokinin-2 receptor PET/CT using the 68Ga-labeled minigastrin analog DOTA-MGS5 in patients with medullary thyroid cancer. JOURNAL OF NUCLEAR MEDICINE, 2023;64(6):859-862.
- von Guggenberg, E., di Santo, G., Uprimny, C., Bayerschmidt, S., Warwitz, B., Hörmann, A. A., Zavvar, T. S., Rangger, C., Decristoforo, C., Sviridenko, A., Nilica, B., Santo, G., & Virgolini, I. J. Safety, biodistribution, and radiation dosimetry of the 68Ga-labeled minigastrin analog DOTA-MGS5 in patients with advanced medullary thyroid cancer and other neuroendocrine tumors. JOURNAL OF NUCLEAR MEDICINE, 2025 Feb 3;66(2):257-263. doi: 10.2967/jnumed.124.268877.
- Zavvar, T. S., Hörmann, A. A., Konijnenberg, M., Kraihammer, M., Mair, C., Kronthaler, A., Joosten, L., Laverman, P., Gruber, L., di Santo, G., Decristoforo, C., Virgolini, I., & von Guggenberg, E. Radiopharmaceutical formulation and preliminary clinical dosimetry of [177Lu]Lu-DOTA-MGS5 for application in peptide receptor radionuclide therapy. EUROPEAN JOURNAL OF NUCLEAR MEDICINE AND MOLECULAR IMAGING, 2025 Mar;52(4):1321-1331. doi: 10.1007/s00259-024-06979-1.
- Di Santo, G., Santo, G., Martinovic, V., Wolf, D., Pircher, A., Sviridenko, A., Löffler-Ragg, J., von Guggenberg, E., & Virgolini, I. Cholecystokinin-2 receptor targeting by [68Ga]Ga-DOTA-MGS5 PET/CT in a patient with extensive disease small cell lung cancer. European Journal of Nuclear Medicine and Molecular Imaging, 2024 Jul;51(9):2848-2849. doi: 10.1007/s00259-024-06749-z.
- Zierke, M. A., Rangger, C., Samadikhah, K., Schmid, A. M., & Haubner, R. 68Ga-labeled glycopeptides as effective tools for liver function imaging. MOLECULAR PHARMACEUTICS, 2025;22(3):1677-1685.
- Zierke, M. A., Rangger, C., Samadikhah, K., Panzer, M., Dichtl, S., Hoermann, N., Wilflingseder, D., Schmid, A. M., & Haubner, R. [68Ga]Ga-NODAGA-TriGalactan, a low molecular weight tracer for the non-invasive imaging of the functional liver reserve. EJNMMI RADIOPHARMACY AND CHEMISTRY, 2024;9(1):41.
- Opalinska, M., Lezaic, L., Decristoforo, C., Kolenc, P., Mikolajczak, R., Studen, A., Simoncic, U., Virgolini, I., Trofimiuk-Muldner, M., Garnuszek, P., Rangger, C., Fani, M., Glowa, B., Skorkiewicz, K., & Hubalewska-Dydejczyk, A. Comparison of 99mTc radiolabeled somatostatin antagonist with [68Ga]Ga-DOTA-TATE in a patient with advanced neuroendocrine tumor. EUROPEAN JOURNAL OF NUCLEAR MEDICINE AND MOLECULAR IMAGING, 2023;50(13):4110-4111.
- von Eyben, R., Hoffmann, M. A., Soydal, C., Virgolini, I., Tuncel, M., Gauthe, M., Kapp, D. S., & von Eyben, F. E. Pretest PSA and restaging PSMA PET/CT predict survival in biochemically recurrent prostate cancer. BIOMEDICINES, 2023;11(9):2333. doi: 10.3390/biomedicines11092333.
- Hoffmann, M. A., Soydal, C., Virgolini, I., Tuncel, M., Kairemo, K., Kapp, D. S., & von Eyben, F. E. Management based on pretreatment PSMA PET of patients with localized high-risk prostate cancer part 2: Prediction of recurrence—A systematic review and meta-analysis. CANCERS, 2025 Feb 28;17(5):841. doi: 10.3390/cancers17050841.
- von Eyben, F. E., Virgolini, I., & Baum, R. Review on the increasing role for PSMA-based radioligand therapy in prostate cancer. CANCERS, 2024;16(14):2520. doi: 10.3390/cancers16142520
- Santo, G., Di Santo, G., Sviridenko, A., Bayerschmidt, S., Wirth, L., Scherbauer, F., Lehmann, P., von Guggenberg, E., Decristoforo, C., Heidegger-Pircher, I., Bektic, J., & Virgolini, I. Efficacy and safety of rechallenge with [177Lu]Lu-PSMA-I&T radioligand therapy in metastatic castration resistant prostate cancer. EUROPEAN JOURNAL OF NUCLEAR MEDICINE AND MOLECULAR IMAGING, 2024;52(1):354-365.
- Giannini, G., Kafka, M., Neuwirt, H., Artamonova, N., Santo, G. D., Virgolini, I., Dotzauer, R., Deiss, E., Paffenholz, P., Heidenreich, A., Rasul, S., Tsaur, I., Rausch, S., Einspieler, H., la Fougère, C., Trautwein, N. F., Zattoni, F., Sepulcri, M., & Heidegger, I. Safety and efficacy of 177Lu-PSMA therapy following 223Radium treatment: A retrospective multinational real-world analysis. Clinical Genitourinary Cancer, 2025 Feb;23(1):102260. doi: 10.1016/j.clgc.2024.102260.
- Laudicella, R., Bauckneht, M., Burger, I. A., Cacciola, A., Fanti, S., Farolfi, A., Ficarra, V., Iagaru, A., Liberini, V., Pergolizzi, S., Santo, G., Virgolini, I., Minutoli, F., & Baldari, S. The role of PSMA-based radioligand therapy in hormone-sensitive prostate cancer. European Journal of Nuclear Medicine and Molecular Imaging, 2025 Feb 12. doi: 10.1007/s00259-025-07083-8.
- Kafka, M., Horninger, A., di Santo, G., Virgolini, I., Neuwirt, H., Unterrainer, L. M., Kunte, S. C., Deiss, E., Paffenholz, P., Heidenreich, A., Rasul, S., Einspieler, H., Shariat, S. F., Rajwa, P., Dozauer, R., Tsaur, I., Medlock, E., Rölz, N., Rausch, S., la Fougère, C., Trautwein, N., Roesch, M. C., Merseburger, A. S., Zattoni, F., Sepulcri, M., Ladurner, M., Bektic, J., Gandaglia, G., Horninger, W., & Heidegger, I. Real-world outcomes and predictive biomarkers for 177Lutetium prostate-specific membrane antigen ligand treatment in metastatic castration-resistant prostate cancer: A European Association of Urology Young Academic Urologists Prostate Cancer Working Group multi-institutional observational study. European Urology Oncology, 2024 Jun;7(3):421-429. doi: 10.1016/j.euo.2023.07.018.
- Kraihammer, M., von Guggenberg, E., Hoermann, A. A., Gabriel, M., & Decristoforo, C. Automated production of [68Ga]Ga-DOTA-exendin-4 via fractionated radionuclide generator elution on a cassette-based synthesis module. NUCLEAR MEDICINE AND BIOLOGY, 2023;124-125:108381.
- Di Santo, G., Santo, G., Sviridenko, A., & Virgolini, I. Peptide receptor radionuclide therapy combinations for neuroendocrine tumours in ongoing clinical trials: Status 2023. THERANOSTICS, 2024;14(3):940-953.
- Santo, G., Di Santo, G., & Virgolini, I. Peptide receptor radionuclide therapy of neuroendocrine tumors: Agonist, antagonist, and alternatives. Seminars in Nuclear Medicine, 2024 Jul;54(4):557-569. doi: 10.1053/j.semnuclmed.2024.02.002.
- Santo, G., di Santo, G., & Cicone, F. Peptide receptor radionuclide therapy with somatostatin analogs beyond gastroenteropancreatic neuroendocrine tumors. Journal of Neuroendocrinology, 2025;37(3):e70013. doi:10.1111/jne.70013.
- Sviridenko, A., di Santo, G., & Virgolini, I. Imaging fibrosis. PET CLINICS, 2023;18(3):381-388.
- Kraihammer, M., Garnuszek, P., Bauman, A., Maurin, M., Alejandre Lafont, M., Haubner, R., von Guggenberg, E., Gabriel, M., & Decristoforo, C. Improved quality control of [177Lu]Lu-PSMA I&T. EJNMMI RADIOPHARMACY AND CHEMISTRY, 2023;8(1):7.
Selection of Funding
Patents
- European Patent Application: New PET Tracer for Imaging of the Functional Hepatic Reserve; PCT/EP2023/075302
- European Patent Application: Peptide-based radiopharmaceuticals for non-invasive imaging of the functional liver reserve; EP24171634.9
Funding
- FWF (P30924-B26): Modified Siderophores for “Theranostics” of Aspergillosis, 2018, Prof.h.c. Dr. Clemens DECRISTOFORO
- FWF (KLI 909-B): 68Ga-DFO based PET-Imaging of Infections, 2021, Prof.h.c. Dr. Clemens DECRISTOFORO
- FWF (I4229-B): Novel 99mTc-labeled somatostatin receptor antagonists, 2019, Univ.-Prof. Dr. Irene VIRGOLINI
- PRISMAP (European medical isotope programme): Production of high purity isotopes by mass separation EU-INFRAIA, H2020, March 2021-February 2025, Prof.h.c. Dr. Clemens DECRISTOFORO
- DOC-Funds: Image-guided Diagnosis and Therapy (IGDT): Integrating multimodal strategies for clinical research, 2021-2025, Prof.h.c. Dr. Clemens DECRISTOFORO, Priv.-Doz. Dr. Elisabeth VON GUGGENBERG
- FWF (P34732): Exploration of innovative radiolabelling strategies for high sensitivity PET imaging of cholecystokinin-2-receptor expressing tumours Innovative radiolabelling strategies for CCK2R targeting, 2021-2024, Priv.-Doz. Dr. Elisabeth VON GUGGENBERG
- FWF (P34802): New PET tracer for imaging of functional liver, since 2021, Dr. Roland HAUBNER
Collaborations
- Andrew Iagaru, Professor of Radiology/Nuclear Medicine & Chief of the Division of Nuclear Medicine and Molecular Imaging, Stanford University Medical Centre, California, USA
- Hubert Hass, Institute of Molecular Biology, Biocentre, University of Innsbruck, AUT
- Dominic Wolf, Department of Oncology, Medical University of Innsbruck. AUT
- Dr. Stefano FANTI, Sant’Orsola-Malpighi Polyclinic, Bologna, ITA
- Prof. Dr. Sabina DIZDAREVIC, University Hospitals Sussex NHS Foundation Trust, Sussex, GBR
- Prof. Dr. Mike SATHEKGE, University of Pretoria and Steve Biko Academic Hospital, Pretoria, ZAF
- Ass.-Prof. Miloš PETRIK PharmDr., Ph.D., Institute of Molecular and Translational Medicine, Faculty of Medicine and Dentistry, Palacký University Olomouc, Olomouc, CZE
- Prof. Renata MIKOLAJCAK, Radioisotope Centre POLATOM, National Centre for Nuclear Research, Otwock, POL
- Prof. Dr. Alicja HUBALEWSKA-DYDEJCZYK, Department of Endocrinology, Jagiellonian University Medical College, Cracow, POL
- Dr. Jane SOSABOWSKI, PhD, Centre for Molecular Oncology, Barts Cancer Institute, Queen Mary University of London, London, GBR
- Dr. Peter LAVERMAN, Radboud University Medical Centre, Nijmegen, NLD
- Prof. Dr. Elzbieta GUMIENNA-KONTECKA, Department of Inorganic Chemistry, University of Wroclaw, Wroclaw, POL
- Prof. Dr. Bernd PICHLER/Dr. Andreas SCHMID, Werner Siemens Imaging Centre, Medizinische Universität Tübingen, Tübingen, GER
- Prof. Dr. Thomas Beyer, Medical University of Vienna, AUT